Discovery of the Periodic Table of Chemical Elements e. and
In the winter of 1867-68, Mendeleev began to write the textbook "Fundamentals of Chemistry" and immediately encountered difficulties in systematizing the factual material. By mid-February 1869, while pondering the structure of the textbook, he gradually came to the conclusion that the properties of simple substances (and this is the form of the existence of chemical elements in a free state) and the atomic masses of elements are connected by a certain pattern.
Mendeleev did not know much about the attempts of his predecessors to arrange the chemical elements in order of increasing atomic masses and about the incidents that arose in this case. For example, he had almost no information about the work of Chancourtois, Newlands, and Meyer.
The decisive stage of his thoughts came on March 1, 1869 (February 14, old style). A day earlier, Mendeleev wrote a request for a ten-day vacation to inspect artel cheese factories in the Tver province: he received a letter with recommendations for studying cheese production from A. I. Khodnev, one of the leaders of the Free Economic Society.
Petersburg that day was cloudy and frosty. The trees creaked in the wind in the university garden, where the windows of Mendeleev's apartment looked out. While still in bed, Dmitry Ivanovich drank a mug of warm milk, then got up, washed himself and went to breakfast. His mood was wonderful.
At breakfast, Mendeleev had an unexpected idea: to compare close atomic masses of various chemical elements and their chemical properties.
Without thinking twice, on the reverse side of Khodnev's letter, he wrote down the symbols for chlorine Cl and potassium K with fairly similar atomic masses, equal to 35.5 and 39, respectively (the difference is only 3.5 units). In the same letter, Mendeleev sketched symbols of other elements, looking for similar "paradoxical" pairs among them: fluorine F and sodium Na, bromine Br and rubidium Rb, iodine I and cesium Cs, for which the mass difference increases from 4.0 to 5.0 and then to 6.0. Mendeleev then could not know that the "indefinite zone" between obvious non-metals and metals contains elements - noble gases, the discovery of which in the future will significantly modify the Periodic Table.
After breakfast, Mendeleev closed himself in his office. He took out a pack of business cards from the desk and began to write the symbols of the elements and their main chemical properties on their reverse side.
After a while, the household heard how it began to be heard from the office: "Uuu! Horned one. Wow, what a horned one! I will overcome them. I will kill them!" These exclamations meant that Dmitry Ivanovich had a creative inspiration.
Mendeleev shifted the cards from one horizontal row to another, guided by the values of the atomic mass and the properties of simple substances formed by atoms of the same element. Once again, a thorough knowledge of inorganic chemistry came to his aid. Gradually, the appearance of the future Periodic Table of chemical elements began to take shape.
So, at first he put a card with the element beryllium Be (atomic mass 14) next to the card of the aluminum element Al (atomic mass 27.4), according to the then tradition, taking beryllium for an analog of aluminum. However, then, comparing the chemical properties, he placed beryllium over magnesium Mg. Having doubted the then generally accepted value of the atomic mass of beryllium, he changed it to 9.4, and changed the formula of beryllium oxide from Be2O3 to BeO (like magnesium oxide MgO). By the way, the "corrected" value of the atomic mass of beryllium was confirmed only ten years later. He acted just as boldly on other occasions.
Gradually, Dmitry Ivanovich came to the final conclusion that the elements, arranged in ascending order of their atomic masses, show a clear periodicity in physical and chemical properties.
Throughout the day, Mendeleev worked on the system of elements, taking short breaks to play with his daughter Olga, have lunch and dinner.
On the evening of March 1, 1869, he whitewashed the table he had compiled and, under the title "Experiment of a system of elements based on their atomic weight and chemical similarity," sent it to the printer, making notes for typesetters and putting the date "February 17, 1869" (according to the old style ).
This is how the Periodic Law was discovered, the modern formulation of which is as follows: “The properties of simple substances, as well as the forms and properties of compounds of elements, are in a periodic dependence on the charge of the nuclei of their atoms”
Mendeleev was then only 35 years old.
Mendeleev sent printed sheets with a table of elements to many domestic and foreign chemists, and only after that he left St. Petersburg to inspect cheese factories.
Before his departure, he still managed to hand over to N. A. Menshutkin, an organic chemist and future historian of chemistry, the manuscript of the article "Relationship of properties with the atomic weight of elements" - for publication in the Journal of the Russian Chemical Society and for communication at the upcoming meeting of the society.
On March 18, 1869, Menshutkin, who at that time was the clerk of the society, made a small report on the Periodic Law on behalf of Mendeleev. The report at first did not attract much attention of chemists, and the President of the Russian Chemical Society, Academician Nikolai Zinin (1812-1880) stated that Mendeleev was not doing what a real researcher should do. True, two years later, after reading Dmitry Ivanovich's article "The natural system of elements and its application to indicating the properties of certain elements," Zinin changed his mind and wrote to Mendeleev: "Very, very good, very excellent approximations, even fun to read, God bless you good luck in experimental confirmation of your conclusions. Sincerely devoted to you and deeply respecting you N. Zinin.
Mendeleev still had a lot to do after the discovery of the Periodic Law. The reason for the periodic change in the properties of the elements remained unknown, and the very structure of the Periodic Table, where the properties were repeated through seven elements in the eighth, did not find an explanation. However, the first veil of mystery was removed from these numbers: in the second and third periods of the system, there were then just seven elements each.
Mendeleev did not place all the elements in ascending order of atomic masses; in some cases he was more guided by the similarity of chemical properties. So, cobalt Co has an atomic mass greater than nickel Ni, tellurium Te also has a greater atomic mass than iodine I, but Mendeleev placed them in the order Co - Ni, Te - I, and not vice versa. Otherwise, tellurium would fall into the group of halogens, and iodine would become a relative of selenium Se.
The most important thing in the discovery of the Periodic Law is the prediction of the existence of yet undiscovered chemical elements. Under aluminum Al, Mendeleev left a place for its analogue "ekaaluminum", under boron B - for "ekabor", and under silicon Si - for "ekasilicon". This is how Mendeleev called chemical elements that had not yet been discovered. He even gave them the symbols El, Eb and Es.
Regarding the element "ecasilicon", Mendeleev wrote: "It seems to me that the most interesting of the undoubtedly missing metals will be the one that belongs to the IV group of analogues of carbon, namely, to the III series. This will be the metal immediately following silicon, and therefore we will name his exacilitation." Indeed, this as yet undiscovered element was supposed to become a kind of "lock" connecting two typical non-metals - carbon C and silicon Si - with two typical metals - tin Sn and lead Pb.
Not all foreign chemists immediately appreciated the significance of Mendeleev's discovery. It changed a lot in the world of established ideas. Thus, the German physical chemist Wilhelm Ostwald, the future Nobel Prize winner, argued that it was not the law that was discovered, but the principle of classifying "something indefinite". The German chemist Robert Bunsen, who discovered two new alkaline elements in 1861, rubidium Rb and cesium Cs, wrote that Mendeleev was taking chemists "into a far-fetched world of pure abstractions."
Hermann Kolbe, a professor at the University of Leipzig, called Mendeleev's discovery "speculative" in 1870. Kolbe was distinguished by rudeness and rejection of new theoretical views in chemistry. In particular, he was an opponent of the theory of the structure of organic compounds and at one time sharply attacked Jacob van't Hoff's article "Chemistry in Space". Van't Hoff later became the first Nobel laureate for his research. But Kolbe suggested that such researchers as van't Hoff "exclude from the ranks of real scientists and enroll them in the camp of spiritualists"!
Every year the Periodic Law won more and more supporters, and its discoverer - more and more recognition. High-ranking visitors began to appear in Mendeleev's laboratory, including even Grand Duke Konstantin Nikolayevich, head of the naval department.
Here the reader will find information about one of the most important laws ever discovered by man in the scientific field - the periodic law of Mendeleev Dmitry Ivanovich. You will get acquainted with its meaning and influence on chemistry, the general provisions, characteristics and details of the periodic law, the history of discovery and the main provisions will be considered.
What is the periodic law
The periodic law is a natural law of a fundamental nature, which was first discovered by D. I. Mendeleev back in 1869, and the discovery itself was due to a comparison of the properties of some chemical elements and the atomic mass values known at that time.
Mendeleev argued that, according to his law, simple and complex bodies and various compounds of elements depend on their dependence of the periodic type and on the weight of their atom.
The periodic law is unique in its kind and this is due to the fact that it is not expressed by mathematical equations, unlike other fundamental laws of nature and the universe. Graphically, it finds its expression in the periodic table of chemical elements.
Discovery history
The discovery of the periodic law took place in 1869, but attempts to systematize all known x elements began long before that.
The first attempt to create such a system was made by I. V. Debereiner in 1829. He classified all the chemical elements known to him into triads, interconnected by the proximity of half the sum of the atomic masses included in this group of three components. Following Debereiner, an attempt was made to create a unique table of classification of elements by A. de Chancourtois, he called his system the "earth spiral", and after him the Newlands octave was compiled by John Newlands. In 1864, almost simultaneously, William Olding and Lothar Meyer published independently created tables.

The periodic law was presented to the scientific community for review on March 8, 1869, and this happened during a meeting of the Russian X-th Society. Mendeleev Dmitry Ivanovich announced his discovery in front of everyone, and in the same year Mendeleev's textbook "Fundamentals of Chemistry" was published, where the periodic table created by him was shown for the first time. A year later, in 1870, he wrote an article and submitted it for review to the RCS, where the concept of the periodic law was first used. In 1871, Mendeleev gave an exhaustive description of his research in his famous article on the periodic validity of chemical elements.

An invaluable contribution to the development of chemistry
The value of the periodic law is incredibly great for the scientific community around the world. This is due to the fact that its discovery gave a powerful impetus to the development of both chemistry and other natural sciences, such as physics and biology. The relationship of the elements with their qualitative chemical and physical characteristics was open, and this also made it possible to understand the essence of building all the elements according to one principle and gave rise to the modern formulation of the concepts of chemical elements, to concretize the knowledge of the idea of substances of complex and simple structure.
The use of the periodic law made it possible to solve the problem of chemical prediction, to determine the cause of the behavior of known chemical elements. Atomic physics, including nuclear energy, became possible as a result of the same law. In turn, these sciences made it possible to expand the horizons of the essence of this law and delve into its understanding.
Chemical properties of the elements of the periodic system
In fact, the chemical elements are interconnected by the characteristics inherent in them in the state of both a free atom and an ion, solvated or hydrated, in a simple substance and in the form that their numerous compounds can form. However, x-th properties usually consist in two phenomena: properties characteristic of an atom in a free state, and a simple substance. This kind of properties includes many of their types, but the most important are:
- Atomic ionization and its energy, depending on the position of the element in the table, its ordinal number.
- The energy relationship of the atom and electron, which, like atomic ionization, depends on the location of the element in the periodic table.
- The electronegativity of an atom, which does not have a constant value, but can change depending on various factors.
- The radii of atoms and ions - here, as a rule, empirical data are used, which is associated with the wave nature of electrons in a state of motion.
- Atomization of simple substances - a description of the ability of an element to reactivity.
- The oxidation states are a formal characteristic, however, appearing as one of the most important characteristics of an element.
- The oxidation potential for simple substances is a measurement and indication of the potential of a substance to act in aqueous solutions, as well as the level of manifestation of redox properties.

Periodicity of elements of internal and secondary type
The periodic law gives an understanding of another important component of nature - internal and secondary periodicity. The aforementioned fields of study of atomic properties are, in fact, much more complex than one might think. This is due to the fact that the elements s, p, d of the table change their qualitative characteristics depending on their position in the period (internal periodicity) and group (secondary periodicity). For example, the internal process of the transition of the element s from the first group to the eighth to the p-element is accompanied by minimum and maximum points on the energy curve of the ionized atom. This phenomenon shows the internal inconstancy of the periodicity of changes in the properties of an atom according to its position in the period.

Results
Now the reader has a clear understanding and definition of what Mendeleev's periodic law is, realizes its significance for man and the development of various sciences, and has an idea of its current provisions and the history of discovery.
In the book of the prominent Soviet historian of chemistry N.F. Figurovsky "Essay on the General History of Chemistry. The Development of Classical Chemistry in the 19th Century" (M., Nauka, 1979). the main periods of the discovery of 63 chemical elements from ancient times to 1869 - the year of the establishment by Dmitry Ivanovich Mendeleev (1834-1907) of the Periodic Law are given:
1. The most ancient period (from the 5th millennium BC to 1200 AD).
This long period includes the acquaintance of a person with 7 metals of antiquity - gold, silver, copper, lead, tin, iron and mercury. In addition to these elementary substances, sulfur and carbon were known in antiquity, occurring in nature in a free state.
2. Alchemical period.

During this period (from 1200 to 1600), the existence of several elements was established, isolated either in the process of alchemical searches for ways to transmute metals, or in the processes of metal production and processing of various ores by artisan metallurgists. These include arsenic, antimony, bismuth, zinc, phosphorus.
3. The period of emergence and development of technical chemistry (end of the 17th century - 1751).

At that time, as a result of a practical study of the characteristics of various metal ores and overcoming the difficulties that arose in the isolation of metals, as well as discoveries in the process of mineralogical expeditions, the existence of platinum, cobalt, and nickel was established.
4. The first stage of the chemical-analytical period in the development of chemistry (1760-1805). During this period, with the help of qualitative and weight quantitative analyzes, a number of elements were discovered, some of them only in the form of "earths": magnesium, calcium (establishing the difference between lime and magnesia), manganese, barium (barite), molybdenum, tungsten, tellurium, uranium (oxide), zirconium (earth), strontium (earth), titanium (oxide), chromium, beryllium (oxide), yttrium (earth), tantalum (earth), cerium (earth), fluorine (hydrofluoric acid), palladium, rhodium, osmium and iridium.
5. Stage of pneumatic chemistry. At this time (1760-1780), gaseous elements were discovered - hydrogen, nitrogen, oxygen and chlorine (the latter was considered a complex substance - oxidized hydrochloric acid until 1809).
6. The stage of obtaining elements in a free state by electrolysis (G. Davy, 1807-1808) and chemically: potassium, sodium, calcium, strontium, barium and magnesium. All of them, however, were previously known in the form of "flammable" (caustic) alkalis and alkaline earths, or soft alkalis.
7. The second stage of the chemical-analytical period in the development of chemistry (1805-1850). At this time, as a result of improving the methods of quantitative analysis and developing a systematic course of qualitative analysis, boron, lithium, cadmium, selenium, silicon, bromine, aluminum, iodine, thorium, vanadium, lanthanum (earth), erbium (earth), terbium (earth) were discovered. ), ruthenium, niobium.
8. The period of discovery of elements by means of spectral analysis, immediately following the development and introduction of this method into practice (1860-1863): cesium, rubidium, thallium and indium.

As you know, the first in the history of chemistry "Table of Simple Bodies" was compiled by A. Lavoisier in 1787. All simple substances were divided into four groups: "I. Simple substances presented in all three kingdoms of nature, which can be considered as elements of bodies: 1) light, 2) caloric, 3) oxygen, 4) nitrogen, 5) hydrogen II.Simple non-metallic substances that oxidize and give acids: 1) antimony, 2) phosphorus, 3) coal, 4) muriatic acid radical, 5 ) hydrofluoric acid radical, 6) boric acid radical III.Simple metallic substances that oxidize and give acids: 1) antimony, 2) silver, 3) arsenic, 4) bismuth, 5) cobalt, 6) copper, 7) tin, 8) iron, 9) manganese, 10) mercury, 11) molybdenum, 12) nickel, 13) gold, 14) platinum, 15) lead, 16) tungsten, 17) zinc IV. ) lime (calcareous earth), 2) magnesia (magnesium sulfate base), 3) barite (heavy earth), 4) alumina (clay, alum earth), 5) silica (siliceous earth)".
This table formed the basis of the chemical nomenclature developed by Lavoisier. D. Dalton introduced into science the most important quantitative characteristic of atoms of chemical elements - the relative weight of atoms or atomic weight.
When searching for regularities in the properties of atoms of chemical elements, scientists first of all paid attention to the nature of the change in atomic weights. In 1815-1816. the English chemist W. Prout (1785-1850) published two anonymous articles in the Annals of Philosophy, in which the idea was expressed and substantiated that the atomic weights of all chemical elements are integer (i.e., multiples of the atomic weight of hydrogen, which was then taken equal to unit): "If the views that we have decided to express are correct, then we can almost consider that the primordial matter of the ancients is embodied in hydrogen ...". Prout's hypothesis was very tempting and led to the setting up of many experimental studies in order to determine the atomic weights of chemical elements as accurately as possible.
In 1829, the German chemist I. Debereiner (1780-1849) compared the atomic weights of similar chemical elements: Lithium, Calcium, Chlorine, Sulfur, Manganese, Sodium, Strontium, Bromine, Selenium, Chromium, Potassium, Barium, Iodine, Tellurium, Iron and found that the atomic weight of the middle element is equal to half the sum of the atomic weights of the extreme elements. The search for new triads led L. Gmelin (1788-1853), the author of the world-famous reference guide to chemistry, to the establishment of numerous groups of similar elements and to the creation of their peculiar classification.
In the 60s. In the 19th century, scientists switched to comparing the groups of chemically similar elements themselves. Thus, A. Shancourtois (1820-1886), a professor at the Paris Mining School, arranged all the chemical elements on the surface of a cylinder in ascending order of their atomic weights so that a "helix" was obtained. With this arrangement, similar elements often fell on the same vertical line. In 1865, the English chemist D. Newlands (1838-1898) published a table that included 62 chemical elements. The elements were arranged and numbered in ascending order of atomic weights.
Newlands used numbering to emphasize that every seven elements, the properties of chemical elements are repeated. When discussing in the London Chemical Society in 1866 a new article by Newlands (it was not recommended for publication), Professor J. Foster asked sarcastically: “Have you tried to arrange the elements in alphabetical order of their names and have you noticed any new patterns?
In 1868, the English chemist W. Olding (1829-1921) proposed a table, which, in the author's opinion, demonstrated a regular relationship between all elements.
In 1864, the German professor L. Mayer (1830-1895) compiled a table of 44 chemical elements (out of 63 known).
Assessing this period, D.I. Mendeleev wrote "There is not a single general law of nature that would be based immediately, its approval is always preceded by many forebodings, and the recognition of the law does not come when it is fully realized in all its meaning, but only after the confirmation of its consequences by experiments, which natural scientists must recognize as the highest authority of their considerations and opinions.
In 1868 D.I.Mendeleev began to work on the course "Fundamentals of Chemistry". For the most logical arrangement of the material, it was necessary to somehow classify 63 chemical elements. The first version of the Periodic Table of Chemical Elements was proposed by D.I. Mendeleev in March 1869.

Two weeks later, at a meeting of the Russian Chemical Society, Mendeleev's report "The relationship of properties with the atomic weight of elements" was read out, in which possible principles for the classification of chemical elements were discussed:
1) according to their relation to hydrogen (formulas of hydrides); 2) according to their relation to oxygen (formulas of higher oxygen oxides); 3) by valence; 4) in terms of atomic weight.
Further, during the following years (1869-1871), Mendeleev studied and rechecked those regularities and "inconsistencies" that were noticed in the first version of the "System of Elements". Summing up this work, D.I. Mendeleev wrote: "As the atomic weight increases, the elements first have more and more changeable properties, and then these properties are repeated again in a new order, in a new line and in a number of elements and in the same sequence Therefore, the Law of Periodicity can be formulated as follows: "The properties of the elements, and therefore the properties of the simple and complex bodies they form, are in a periodic dependence (i.e., they repeat correctly) on their atomic weight." nature of exceptions cannot be tolerated... The affirmation of a law is possible only by deriving consequences from it, which are impossible and unexpected without it, and by justifying those consequences and by experimental verification. there are such logical consequences that could show whether it is true or not.These include the prediction of the properties of undiscovered elements and the correction of the atomic weights of many, few studied elements at that time ... One must do one thing - or consider the periodic law to be true to the end and constituting a new instrument of chemical knowledge, or reject it."
During 1872-1874. Mendeleev began to deal with other problems, and there was almost no mention of the Periodic Law in the chemical literature.
In 1875, the French chemist L. de Boisbaudran reported that while studying zinc blende, he spectroscopically discovered a new element in it. He received the salts of this element and determined its properties. In honor of France, he named the new element gallium (as France was called by the ancient Romans). Let's compare what D.I. Mendeleev predicted and what was found by L. de Boisbaudran:
In the first report by L. de Boisbaudran, the specific gravity of gallium was found to be 4.7. DIMendeleev pointed out to him his mistake. A more careful measurement showed that the specific gravity of gallium was 5.96.
In 1879, the Swedish chemist L. Nilsson (1840-1899) reported on the discovery of a new chemical element - scandium. L. Nilson classified scandium as a rare earth element. P.T.Kleve pointed out to L.Nilson that scandium salts are colorless, its oxide is insoluble in alkalis, and that scandium is ekabor predicted by D.I.Mendeleev. Let's compare their properties.
Analyzing a new mineral in February 1886, German professor K. Winkler (1838-1904) discovered a new element and considered it an analogue of antimony and arsenic. There was a discussion. K. Winkler agreed that the element he had discovered was the ecasilicon predicted by D. I. Mendeleev. K. Winkler called this element germanium.
So, chemists confirmed the existence of the chemical elements predicted by Mendeleev three times. Moreover, it was precisely the properties of these elements predicted by Mendeleev and their position in the Periodic system that made it possible to correct the errors that experimenters unwittingly made. The further development of chemistry took place on a solid basis of the Periodic Law, which in the 80s of the XIX century. was recognized by all scientists as one of the most important laws of nature. Thus, the most important characteristic of any chemical element is its place in the Periodic system of D.I. Mendeleev.
In his 1668 work, Robert Boyle provided a list of indecomposable chemical elements. There were only fifteen of them at that time. At the same time, the scientist did not claim that, in addition to the elements he listed, there were no more, and the question of their number remained open.
A hundred years later, the French chemist Antoine Lavoisier compiled a new list of elements known to science. 35 chemicals were included in his registry, of which 23 were subsequently recognized as those very indecomposable elements.
The search for new elements was carried out by chemists all over the world and progressed quite successfully. The decisive role in this issue was played by the Russian chemist Dmitry Ivanovich Mendeleev: it was he who came up with the idea of the possibility of a relationship between the atomic mass of elements and their place in the "hierarchy". In his own words, "it is necessary to look for ... correspondences between the individual properties of elements and their atomic weights."
Comparing the chemical elements known at that time, Mendeleev, after a colossal work, finally discovered that dependence, the general regular connection between the individual elements, in which they appear as a single whole, where the properties of each element are not something that exists by itself, but periodically and a regularly recurring phenomenon.
So in February 1869 it was formulated periodic law of Mendeleev. In the same year, on March 6, a report prepared by D.I. Mendeleev, under the title "Relationship of properties with the atomic weight of elements" was presented by N.A. Menshutkin at a meeting of the Russian Chemical Society.
In the same year, the publication appeared in the German magazine "Zeitschrift für Chemie", and in 1871, a detailed publication by D.I. Mendeleev, dedicated to his discovery - "Die periodische Gesetzmässigkeit der Elemente" (Periodic regularity of chemical elements).
Creating a Periodic Table
Despite the fact that the idea was formed by Mendeleev in a rather short period of time, he could not formalize his conclusions for a long time. It was important for him to present his idea in the form of a clear generalization, a strict and visual system. As D.I. Mendeleev in a conversation with Professor A.A. Inostrantsev: "Everything came together in my head, but I can't express it in a table."
According to biographers, after this conversation, the scientist worked on creating the table for three days and three nights, not going to bed. He went through various options in which elements could be combined to organize in a table. The work was also complicated by the fact that at the time of the creation of the periodic system, not all chemical elements were known to science.
In 1869-1871, Mendeleev continued to develop the ideas of periodicity put forward and accepted by the scientific community. One of the steps was the introduction of the concept of the place of an element in the periodic system as a set of its properties in comparison with the properties of other elements.
It was on the basis of this, and also based on the results obtained in the course of studying the sequence of changes in glass-forming oxides, that Mendeleev corrected the values of the atomic masses of 9 elements, including beryllium, indium, uranium and others.
During the work of D.I. Mendeleev sought to fill in the empty cells of his table. As a result, in 1870 he predicted the discovery of elements unknown at that time to science. Mendeleev calculated atomic masses and described the properties of three elements not yet discovered at that time:
- "ekaaluminum" - discovered in 1875, named gallium,
- "ekabora" - discovered in 1879, named scandium,
- "ekasilicia" - discovered in 1885, named germanium.
His next realized predictions were the discovery of eight more elements, including polonium (discovered in 1898), astatine (discovered in 1942-1943), technetium (discovered in 1937), rhenium (discovered in 1925) and France (discovered in 1939).
In 1900, Dmitry Ivanovich Mendeleev and William Ramsay came to the conclusion that it was necessary to include elements of a special, zero group in the periodic system. Today, these elements are called noble gases (until 1962, these gases were called inert gases).

The principle of organization of the periodic system
In his table, D.I. Mendeleev arranged the chemical elements in rows in order of increasing mass, choosing the length of the rows so that the chemical elements in the same column had similar chemical properties.
Noble gases - helium, neon, argon, krypton, xenon and radon are reluctant to react with other elements and show low chemical activity and therefore are in the far right column.
In contrast, the elements of the leftmost column - lithium, sodium, potassium and others react violently with other substances, the process is explosive. Elements in other columns of the table behave similarly - inside the column, these properties are similar, but vary when moving from one column to another.
The periodic system in its first version simply reflected the state of affairs existing in nature. Initially, the table did not explain in any way why this should be so. And only with the advent of quantum mechanics did the true meaning of the arrangement of elements in the periodic table become clear.
Chemical elements up to uranium (contains 92 protons and 92 electrons) are found in nature. Starting with number 93, there are artificial elements created in the laboratory.
30.09.2015
There are quite a lot of discoveries in world history, thanks to which science reached a new level of development, making another round in its knowledge. These revolutionary achievements completely or partially changed the attitude to the solution of the tasks set, and also forced to more extensively reveal the scientific point of view on what is happening.
The date of discovery of the periodic law is 1896. In his law, D.I. Mendeleev makes us look at the arrangement of elements in a system in a different way, proving that the properties of the elements, their forms, the properties of the compounds of these elements, the properties of the substances they form, whether they are simple or complex, depend on the atomic mass. Almost immediately, he published the first book, Fundamentals of Chemistry, in which the periodic table was also printed.
There were many prerequisites for the law, it did not arise from scratch, many works of various scientists were applied to its emergence. The development of chemistry at the dawn of the 19th century caused many difficulties, since some elements had not yet been discovered, and the atomic masses of already known substances were incorrect. The first decades of this century were marked by such discoveries of the basic laws of chemistry, these include the laws of proportions and volumes, Dulong and Petit, and others.
These discoveries became the basis for the development of various experimental studies. But still, most of the disagreements among the teachings gave rise to confusion in the definition of atomic weights, due to which water, for example, at that time was represented by 4 formulas. To settle disputes, it was decided to convene a Congress to which famous chemists were invited. It took place in 1860, it was on it that Canizzaro read a report on atomic-molecular theory. Scientists also managed to come to unity in terms of atom, molecule and equivalent.
The table of simple substances, which Lavoisier proposed back in 1787, consisted of only 35 elements, and by the end of the 19th century there were already 63 of them. Many scientists also tried to find a relationship between the properties of elements in order to more correctly calculate the atomic weight. In this direction, great success was achieved by the chemist Debereiner, who developed the law of triads. J.B. Dumas and M.I. Pettenekofer successfully discovered the homologous series, also expressing assumptions about the correctness of the relationships among atomic weights.
While some calculated the weight of atoms, others tried to streamline the periodic system. Chemist Odling offers a table of 57 elements, divided into 17 groups, further chemist de Chancourt tries to depict everything in a geometric formula. Along with his screw system, Newlands also has a table. In addition, among the researchers it is worth noting Meyer, who in 1864 published a book with a table consisting of 44 elements. After D.I. Mendeleev published his Periodic Law and System, and the chemist Maillet for a long time made claims for his discovery priority.
All these prerequisites formed the basis of the discovery, while Mendeleev himself, a couple of decades after his discovery, said that he had been thinking about the system for almost 20 years. All the main conclusions and provisions of the law were made by him in his writings by the end of 1871. He found that the numerical values of atomic masses are in a certain pattern, and the properties of the elements are just intermediate data that depend on two neighboring elements from above and below, and simultaneously on two elements of the period on the right and left.
Later D.I. Mendeleev had more than one year to prove his discovery. Its recognition came only much later, when germanium, scandium, and gallium were successfully discovered. By the end of the 19th century, most scientists recognized this law as one of the main laws of nature. Over time, at the beginning of the 20th century, the periodic system underwent minor changes, a zero group was formed with inert gases, and rare earth metals were located in one cell.
Discovery of the Periodic Law [VIDEO]
 Discovery of the periodic law of chemical elements D
Discovery of the periodic law of chemical elements D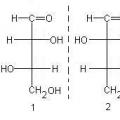 Practical guide to chemistry Fisher's structural formula
Practical guide to chemistry Fisher's structural formula Transcription in biology - what is it?
Transcription in biology - what is it?