Discoveries of M. Planck, N
Max Planck's brief biography of the German physicist is presented in this article.
Max Planck short biography
Max Karl Ernst Ludwig Planck was born in April 23, 1858 in the town of Kilev. His father was a professor of civil law. From a very young age, the boy began to show extraordinary musical abilities, learning to play the piano and organ.
In 1867 his family moved to live in Munich. Here Max Planck entered the Royal Classical Gymnasium, where he developed an interest in the natural and exact sciences.
In 1874, Planck was faced with a choice - to continue his musical studies or to study physics. He preferred the latter. Max began to study physics and mathematics at the Universities of Berlin and Munich, deepening his knowledge of quantum theory, thermodynamics, probability theory, the theory of thermal radiation, history and methodology of physics.
In 1900, a young scientist formulated the law of energy distribution in the spectrum of a black body, introducing a constant with a functional dimension. Max Planck's formula immediately received experimental confirmation. It was a sensation in science. He created the so-called Planck constant or quantum of action - this is one of the universal constants in physics. And the date December 14, 1900, the day when Max Planck presented a report at the German Physical Society on the theoretical foundations of the law of radiation, became the date of birth of the new quantum theory.
Planck's research on probability theory was also of great importance. The German scientist was one of the first to understand it and persistently supported it. This is where his scientific achievements continue - in 1906, Max Planck derived an equation for relativistic dynamics, obtaining in the course of his research formulas for determining the momentum and energy of the electron. Thus, scientists completed the relativization of classical mechanics.
In 1919, Max Planck received the Nobel Prize in Physics for 1918. The list of his achievements included the following - “as a sign of the weight of his merits in the development of physics through the discovery of energy quanta.”
Despite great achievements in science, Planck's personal life was very tragic. His first wife died early, leaving him with 4 children - two daughters and two sons. He married a second time and the scientist’s fifth child was born – a boy. His eldest son died during the First World War, and his two daughters died during childbirth. His second son was executed for participating in the assassination attempt on Hitler.
Max Planck died in Göttingen October 4, 1947 just six months short of his 90th birthday.
Planck, who was her creator and how important she became for the development of modern science. The importance of the idea of quantization for the entire microworld is also shown.
Smartphone and quantum physics
The modern world around us is very different in technology from everything that was familiar a hundred years ago. All this became possible only because at the dawn of the twentieth century, scientists overcame the barrier and finally understood: matter on the smallest scale is not continuous. And this era was opened by a remarkable man - Max Planck.
Biography of Planck
One of the physical constants, a quantum equation, the scientific community in Germany, an asteroid, and a space telescope are named after him. His image was embossed on coins and printed on stamps and banknotes. What kind of person was Max Planck? He was born in the mid-nineteenth century into a poor German noble family. Among his ancestors there were many good lawyers and church ministers. M. Planck received a good education, but fellow physicists jokingly called him “self-taught.” The scientist received his basic knowledge from books.
Planck's hypothesis was born from an assumption that he derived theoretically. In his scientific career, he adhered to the principle of “science comes first.” During the First World War, Planck tried to maintain connections with foreign colleagues from Germany’s enemy countries. The arrival of the Nazis found him in the position of director of a large scientific community - and the scientist sought to protect his employees and helped those who fled the regime to go abroad. So Planck's hypothesis was not the only thing for which he was respected. However, he never openly spoke out against Hitler, apparently realizing that he would not only harm himself, but also would not be able to help those who needed it. Unfortunately, many physicists did not accept this position of M. Planck and stopped corresponding with him. He had five children, and only the youngest survived his father. The eldest son was taken away by the First World War, the middle one by the Second World War. Both daughters did not survive childbirth. At the same time, contemporaries noted that only at home Planck was himself.
Sources of quanta
Since school, the scientist has been interested in it. It says: any process occurs only with an increase in chaos and a loss of energy or mass. He was the first to formulate it exactly like that - in terms of entropy, which can only increase in a thermodynamic system. Later, it was this work that led to the formulation of the famous Planck hypothesis. He was also one of those who introduced the tradition of separating mathematics and physics, practically creating the theoretical section of the latter. Before him, all natural sciences were mixed, and experiments were carried out by individuals in laboratories that were almost no different from alchemical ones.
Quantum hypothesis

Exploring the entropy of electromagnetic waves in terms of oscillators and relying on experimental data obtained two days earlier, on October 19, 1900, Planck presented to other scientists the formula that would later be named after him. It related the energy, wavelength and temperature of radiation (in the limiting case for All the next night, his colleagues under the leadership of Rubens carried out experiments to confirm this theory. And it turned out to be correct! However, in order to theoretically substantiate the hypothesis arising from this formula and at the same time avoid mathematical complexities such as infinities, Planck had to admit that energy is not emitted in a continuous stream, as previously thought, but in separate portions (E = hν). This approach destroyed all existing ideas about a solid body. Planck's quantum hypothesis revolutionized physics.
Consequences of quantization

At first, the scientist did not realize the importance of his discovery. For some time, the formula he derived was used only as a convenient way to reduce the number of mathematical operations for calculation. At the same time, both Planck and other scientists used continuous Maxwell equations. The only thing that confused me was the constant h, which could not be given a physical meaning. Later, only Albert Einstein and Paul Ehrenfest, understanding the new phenomena of radioactivity and trying to find a mathematical basis for optical spectra, understood the importance of what Planck's hypothesis is. They say that the report at which the formula was presented for the first time opened the era of new physics. Einstein was probably the first to recognize its beginnings. So this is his merit too.
What is quantized
All states that any elementary particle can assume are discrete. A trapped electron can only be at certain levels. The excitation of an atom, like the opposite process - emission, also occurs in jumps. Any electromagnetic interactions are an exchange of quanta of the corresponding energy. Humanity has harnessed the energy of the atom only thanks to the understanding of discreteness. We hope that now readers will not have a question about what Planck’s hypothesis is, and what is its impact on the modern world, and therefore on each of the people.
German physicist Max Karl Ernst Ludwig Planck was born in Kiel (which then belonged to Prussia), in the family of Johann Julius Wilhelm von Planck, professor of civil law, and Emma (nee Patzig) Planck. As a child, the boy learned to play the piano and organ, revealing extraordinary musical abilities. In 1867, the family moved to Munich, and there P. entered the Royal Maximilian Classical Gymnasium, where an excellent mathematics teacher first aroused his interest in the natural and exact sciences. After graduating from high school in 1874, he was going to study classical philology, tried his hand at musical composition, but then gave preference to physics.
For three years P. studied mathematics and physics at the University of Munich and a year at the University of Berlin. One of his professors in Munich, experimental physicist Philipp von Jolly, turned out to be a bad prophet when he advised young P. to choose another profession, since, according to him, there was nothing fundamentally new left in physics that could be discovered. This point of view, widespread at that time, arose under the influence of the extraordinary successes of scientists in the 19th century. have achieved in increasing our knowledge of physical and chemical processes.
While in Berlin, P. acquired a broader view of physics thanks to the publications of outstanding physicists Hermann von Helmholtz and Gustav Kirchhoff, as well as articles by Rudolf Clausius. Familiarity with their works contributed to the fact that P.'s scientific interests focused for a long time on thermodynamics - a field of physics in which, on the basis of a small number of fundamental laws, the phenomena of heat, mechanical energy and energy conversion are studied. P. received his academic degree as a doctor in 1879, having defended a dissertation at the University of Munich on the second law of thermodynamics, which states that no continuous self-sustaining process can transfer heat from a colder body to a warmer one.
The next year, P. wrote another work on thermodynamics, which brought him the position of junior assistant at the Faculty of Physics at the University of Munich. In 1885 he became an associate professor at the University of Kiel, which strengthened his independence, strengthened his financial position and provided more time for scientific research. P.'s work on thermodynamics and its applications to physical chemistry and electrochemistry earned him international recognition. In 1888, he became an associate professor at the University of Berlin and director of the Institute of Theoretical Physics (the post of director was created specifically for him). He became a full (full) professor in 1892.
Since 1896, P. became interested in measurements made at the State Institute of Physics and Technology in Berlin, as well as in the problems of thermal radiation of bodies. Any body containing heat emits electromagnetic radiation. If the body is hot enough, then this radiation becomes visible. As the temperature rises, the body first becomes red-hot, then orange-yellow, and finally white. Radiation emits a mixture of frequencies (in the visible range, the frequency of radiation corresponds to color). However, the radiation of a body depends not only on temperature, but also to some extent on surface characteristics such as color and structure.
Physicists have adopted an imaginary absolute black body as an ideal standard for measurement and theoretical research. By definition, a completely black body is a body that absorbs all radiation incident on it and does not reflect anything. The radiation emitted by a black body depends only on its temperature. Although such an ideal body does not exist, a closed shell with a small opening (for example, a properly constructed oven whose walls and contents are in equilibrium at the same temperature) can serve as an approximation.
One of the proofs of the black-body characteristics of such a shell comes down to the following. Radiation incident on the hole enters the cavity and, reflecting from the walls, is partially reflected and partially absorbed. Since the probability that the radiation will come out through the hole as a result of numerous reflections is very small, it is almost completely absorbed. The radiation originating in the cavity and emerging from the hole is generally considered to be equivalent to the radiation emitted by a hole-sized area on the surface of a black body at the temperature of the cavity and shell. Preparing his own research, P. read Kirchhoff's work on the properties of such a shell with a hole. An accurate quantitative description of the observed distribution of radiation energy in this case is called the black body problem.
As blackbody experiments have shown, a graph of energy (brightness) versus frequency or wavelength is a characteristic curve. At low frequencies (long wavelengths), it is pressed against the frequency axis, then at some intermediate frequency it reaches a maximum (a peak with a rounded top), and then at higher frequencies (short wavelengths) it decreases. As the temperature increases, the curve retains its shape, but shifts toward higher frequencies. Empirical relationships have been established between temperature and the frequency of the peak in the black body radiation curve (Wien's displacement law, named after Wilhelm Wien) and between temperature and the total radiated energy (Stefan–Boltzmann law, named after the Austrian physicists Joseph Stefan and Ludwig Boltzmann ), but no one was able to derive the black body radiation curve from the first principles known at the time.
Wien managed to obtain a semi-empirical formula that can be adjusted so that it describes the curve well at high frequencies, but incorrectly conveys its behavior at low frequencies. J. W. Strett (Lord Rayleigh) and the English physicist James Jeans applied the principle of equal distribution of energy among the frequencies of oscillators contained in the space of a black body, and came to another formula (the Rayleigh-Jeans formula). It reproduced the black body radiation curve well at low frequencies, but diverged from it at high frequencies.
P., under the influence of James Clerk Maxwell's theory of the electromagnetic nature of light (published in 1873 and confirmed experimentally by Heinrich Hertz in 1887), approached the black body problem from the point of view of the distribution of energy between elementary electrical oscillators, the physical form of which was not specified in any way. Although at first glance it may seem that the method he chose resembles the Rayleigh-Jeans conclusion, P. rejected some of the assumptions accepted by these scientists.
In 1900, after long and persistent attempts to create a theory that would satisfactorily explain the experimental data, P. managed to derive a formula that, as experimental physicists from the State Institute of Physics and Technology discovered, agreed with the measurement results with remarkable accuracy. Wien's and Stefan-Boltzmann's laws also followed from Planck's formula. However, to derive his formula, he had to introduce a radical concept that went against all established principles. The energy of Planck oscillators does not change continuously, as would follow from traditional physics, but can only take discrete values, increasing (or decreasing) in finite steps. Each energy step is equal to a certain constant (now called Planck's constant) multiplied by the frequency. Discrete portions of energy were subsequently called quanta. The hypothesis introduced by P. marked the birth of quantum theory, which accomplished a true revolution in physics. Classical physics, as opposed to modern physics, now means “physics before Planck.”
P. was by no means a revolutionary, and neither he himself nor other physicists were aware of the deep meaning of the concept of “quantum”. For P., the quantum was just a means that made it possible to derive a formula that gave satisfactory agreement with the radiation curve of an absolutely black body. He repeatedly tried to reach agreement within the classical tradition, but without success. At the same time, he noted with pleasure the first successes of quantum theory, which followed almost immediately. His new theory included, in addition to Planck's constant, other fundamental quantities, such as the speed of light and a number known as Boltzmann's constant. In 1901, based on experimental data on black body radiation, P. calculated the value of Boltzmann's constant and, using other known information, obtained Avogadro's number (the number of atoms in one mole of an element). Based on Avogadro's number, P. was able to find the electric charge of an electron with remarkable accuracy.
The position of quantum theory was strengthened in 1905, when Albert Einstein used the concept of a photon - a quantum of electromagnetic radiation - to explain the photoelectric effect (the emission of electrons from a metal surface illuminated by ultraviolet radiation). Einstein suggested that light has a dual nature: it can behave both as a wave (as all previous physics convinces us of) and as a particle (as evidenced by the photoelectric effect). In 1907, Einstein further strengthened the position of quantum theory by using the concept of quantum to explain the puzzling discrepancies between theoretical predictions and experimental measurements of the specific heat capacity of bodies - the amount of heat required to raise the temperature of one unit of mass of a solid by one degree.
Another confirmation of the potential power of the innovation introduced by P. came in 1913 from Niels Bohr, who applied quantum theory to the structure of the atom. In Bohr's model, electrons in an atom could only be at certain energy levels determined by quantum limitations. The transition of electrons from one level to another is accompanied by the release of an energy difference in the form of a photon of radiation with a frequency equal to the photon energy divided by Planck's constant. Thus, a quantum explanation was obtained for the characteristic spectra of radiation emitted by excited atoms.
In 1919, P. was awarded the Nobel Prize in Physics for 1918 “in recognition of his services to the development of physics through the discovery of energy quanta.” As stated by A.G. Ekstrand, a member of the Royal Swedish Academy of Sciences, at the award ceremony, “P.’s theory of radiation is the brightest of the guiding stars of modern physical research, and, as far as one can judge, it will still be a long time before the treasures that were obtained by his genius are exhausted.” . In the Nobel lecture given in 1920, P. summed up his work and admitted that “the introduction of quantum has not yet led to the creation of a true quantum theory.”
20s witnessed the development by Erwin Schrödinger, Werner Heisenberg, P.A.M. Dirac and others of quantum mechanics - equipped with the complex mathematical apparatus of quantum theory. P. did not like the new probabilistic interpretation of quantum mechanics, and, like Einstein, he tried to reconcile predictions based only on the principle of probability with classical ideas of causality. His aspirations were not destined to come true: the probabilistic approach survived.
P.'s contribution to modern physics is not limited to the discovery of the quantum and the constant that now bears his name. He was strongly impressed by Einstein's special theory of relativity, published in 1905. The full support provided by P. to the new theory greatly contributed to the acceptance of the special theory of relativity by physicists. Among his other achievements is his proposed derivation of the Fokker-Planck equation, which describes the behavior of a system of particles under the influence of small random impulses (Adrian Fokker is a Dutch physicist who improved the method first used by Einstein to describe Brownian motion - the chaotic zigzag movement of tiny particles suspended in a liquid ). In 1928, at the age of seventy, Planck entered into his mandatory formal retirement, but did not break ties with the Kaiser Wilhelm Society for Basic Sciences, of which he became president in 1930. And on the threshold of his eighth decade, he continued his research activities.
P.'s personal life was marked by tragedy. His first wife, née Maria Merck, whom he married in 1885 and who bore him two sons and two twin daughters, died in 1909. Two years later he married his niece Marga von Hesslin, with whom he he also had a son. P.'s eldest son died in the First World War, and in subsequent years both of his daughters died in childbirth. The second son from his first marriage was executed in 1944 for his participation in a failed plot against Hitler.
As a person of established views and religious beliefs, and simply as a fair person, P., after Hitler came to power in 1933, publicly spoke out in defense of Jewish scientists expelled from their posts and forced to emigrate. At a scientific conference he greeted Einstein, who was anathema by the Nazis. When P., as president of the Kaiser Wilhelm Society for Basic Sciences, paid an official visit to Hitler, he took this opportunity to try to stop the persecution of Jewish scientists. In response, Hitler launched into a tirade against Jews in general. Subsequently, P. became more reserved and remained silent, although the Nazis undoubtedly knew about his views.
As a patriot who loved his homeland, he could only pray that the German nation would regain its normal life. He continued to serve in various German learned societies in the hope of preserving at least some small part of German science and enlightenment from complete destruction. After his home and personal library were destroyed during an air raid on Berlin, P. and his wife tried to find refuge on the Rogetz estate near Magdeburg, where they found themselves between the retreating German troops and the advancing Allied forces. In the end, the Planck couple were discovered by American units and taken to the then safe state of Göttingen.
P. died in Göttingen on October 4, 1947, six months before his 90th birthday. Only his first and last name and the numerical value of Planck's constant are engraved on his tombstone.
Like Bohr and Einstein, P. was deeply interested in philosophical problems related to causality, ethics and free will, and spoke on these topics in print and before professional and lay audiences. Acting as a pastor (but without priesthood) in Berlin, P. was deeply convinced that science complements religion and teaches truthfulness and respect.
Throughout his life, P. carried with him the love of music that flared up in him in early childhood. An excellent pianist, he often played chamber works with his friend Einstein until he left Germany. P. was also a keen mountaineer and spent almost every holiday in the Alps.
In addition to the Nobel Prize, P. was awarded the Copley Medal of the Royal Society of London (1928) and the Goethe Prize of Frankfurt am Main (1946). The German Physical Society named its highest award in honor of him, the Planck Medal, and P. himself was the first recipient of this honorary award. In honor of his 80th birthday, one of the minor planets was named Planckian, and after the end of the Second World War, the Kaiser Wilhelm Society for Basic Sciences was renamed the Max Planck Society. P. was a member of the German and Austrian Academies of Sciences, as well as scientific societies and academies of England, Denmark, Ireland, Finland, Greece, the Netherlands, Hungary, Italy, the Soviet Union, Sweden, Ukraine and the United States.
Quantum theory was born in 1901 when Max Planck proposed a theoretical conclusion about the relationship between the temperature of a body and the radiation emitted by that body, a conclusion that had long eluded other scientists. Like his predecessors, Planck proposed that radiation was emitted by atomic oscillators, but he believed that the energy of the oscillators (and therefore the radiation they emit) existed in the form of small discrete portions, which Einstein called quanta. The energy of each quantum is proportional to the frequency of radiation. Although the formula derived by Planck aroused universal admiration, the assumptions he made remained unclear for some time, since they contradicted classical physics. In 1905 Albert Einstein used quantum theory to explain some aspects of the photoelectric effect - the emission of electrons by the surface of a metal upon which ultraviolet radiation falls. Along the way, Einstein noted an apparent paradox: light, which had long been known to travel as continuous waves, exhibits discrete properties when absorbed and emitted.
About eight years later Niels Bohr extended quantum theory to the atom and explained the frequencies of waves emitted by atoms excited in a flame or electrical discharge. Ernest Rutherford showed that the mass of an atom is almost entirely concentrated in the central nucleus, which carries a positive electric charge and is surrounded at relatively large distances by electrons carrying a negative charge, as a result of which the atom as a whole is electrically neutral.
Bohr suggested that electrons could only be in certain discrete orbits corresponding to different energy levels, and that the “jump” of an electron from one orbit to another, with lower energy, was accompanied by the emission of a photon, the energy of which was equal to the difference in the energies of the two orbits. Frequency, according to Planck's theory, is proportional to the energy of the photon. Thus, Bohr's model of the atom established a connection between the various spectral lines characteristic of the substance emitting radiation and the atomic structure. Despite its initial success, Bohr's model of the atom soon required modifications to resolve discrepancies between theory and experiment. In addition, quantum theory at that stage did not yet provide a systematic procedure for solving many quantum problems. However, it became clear that classical physics is unable to explain the fact that an accelerated electron does not fall on the nucleus, losing energy when emitting electromagnetic waves.
A new essential feature of quantum theory emerged in 1924, when Louis de Broil put forward a radical hypothesis about the wave nature of matter: if electromagnetic waves, such as light, sometimes behave like particles (as Einstein showed), then particles, such as the electron, can behave like waves under certain circumstances. Thus, in the microcosm the boundary between classical particles and classical waves has been erased. In de Broglie's formulation, the frequency corresponding to a particle is related to its energy, as in the case of a photon (a particle of light), but de Broglie's proposed mathematical expression was an equivalent relationship between the wavelength, the mass of the particle, and its speed (momentum). The existence of electron waves was experimentally proven in 1927. Clinton J. Davisson And Lester H. Germer in the United States and George Paget Thomson in England.
This discovery in turn led to the creation in 1933. Ernst Ruska electron microscope.
Impressed by Einstein's comments on de Broglie's ideas Erwin Schrödinger made an attempt to apply the wave description of electrons to the construction of a consistent quantum theory not associated with Bohr's inadequate model of the atom. In a certain sense, he intended to bring quantum theory closer to classical physics, which had accumulated many examples of mathematical descriptions of waves. The first attempt he made in 1925 ended in failure. The speeds of electrons in Schrödinger's theory were close to the speed of light, which required the inclusion of Einstein's special theory of relativity and the significant increase in electron mass predicted by it at very high speeds.
One of the reasons for Schrödinger's failure was that he did not take into account the presence of a specific property of the electron, now known as spin (the rotation of the electron around its own axis like a top, but such a comparison is not entirely correct), about which little was known at that time. Schrödinger made the next attempt in 1926. This time the electron velocities were chosen so small that there was no need to invoke the theory of relativity. The second attempt resulted in the derivation of the Schrödinger wave equation, which provides a mathematical description of matter in terms of the wave function. Schrödinger called his theory wave mechanics. The solutions of the wave equation were in agreement with experimental observations and had a profound influence on the subsequent development of quantum theory. Currently, the wave function underlies the quantum mechanical description of microsystems, similar to Hamilton's equations in classical mechanics.
Not long before Werner Heisenberg , Max Born And Pascual Jordan published another version of quantum theory, called matrix mechanics, which described quantum phenomena using tables of observable quantities. These tables represent mathematical sets ordered in a certain way, called matrices, on which, according to known rules, various mathematical operations can be performed. Matrix mechanics also allowed for agreement with observed experimental data, but unlike wave mechanics, it did not contain any specific reference to spatial coordinates or time. Heisenberg especially insisted on abandoning any simple visual representations or models in favor of only those properties that could be determined from experiment, since, according to his considerations, the microworld has a fundamentally different structure than the macroworld in view of the special role of Planck’s constant, which is insignificant in the world large quantities.
Schrödinger showed that wave mechanics and matrix mechanics are mathematically equivalent. Now known collectively as quantum mechanics, these two theories provided a long-awaited common framework for describing quantum phenomena. Many physicists preferred wave mechanics because its mathematical apparatus was more familiar to them, and its concepts seemed more “physical”; operations on matrices are more cumbersome.
Soon after Heisenberg and Schrödinger developed quantum mechanics, Paul Dirac proposed a more general theory that combined elements of Einstein's special theory of relativity with the wave equation. The Dirac equation applies to particles moving at arbitrary speeds. The spin and magnetic properties of the electron followed from Dirac's theory without any additional assumptions. In addition, Dirac's theory predicted the existence of antiparticles, such as the positron and antiproton, twins of particles with electric charges of opposite signs.
] Executive editor L.S. Polak. Compiled by U.I. Frankfurt.
(Moscow: Publishing House "Nauka", 1975. - Series "Classics of Science")
Scan, processing, format: ???, revision: AAW, mor, 2010
- CONTENT:
From the editor (5).
THERMODYNAMICS
On the principle of increasing entropy. First message (9).
On the principle of increasing entropy. Second message (25).
On the principle of increasing entropy. Third message (36).
On the principle of increasing entropy. Fourth message (69).
Remarks on the Carnot-Clausius principle (102).
Mr. Swinburne and Entropy (106).
Entropy (109).
On the mechanical meaning of temperature and entropy (111).
On the Clausius theorem for irreversible cycles and on the increase of entropy (119).
Toward the kinetic theory of gases. Critical Inquiry (121).
On the absolute entropy of monatomic bodies (123).
Absolute entropy and chemical constant (138).
On the statistical definition of entropy (144).
New statistical definition of entropy (154).
On the potential difference of weak solutions (168).
On the potential difference of weak solutions. Second message (173).
Le Chatelier-Brown principle (177).
Notes on quantity parameter, intensity parameter and stable equilibrium (186).
RADIATION THEORY AND QUANTUM THEORY
On irreversible radiation processes (191).
Entropy and temperature of radiant energy (234).
On one improvement of Wien's radiation law (249).
Towards the theory of distribution of radiation energy of the normal spectrum (251).
On the law of energy distribution in the normal spectrum (258).
About the elementary quantum of matter and electricity (268).
On irreversible radiation processes. Addition (271).
Laws of thermal radiation and the hypothesis of the elementary quantum of action (282).
Modern significance of the quantum hypothesis for the kinetic theory of gases (311).
Modified formulation of the quantum hypothesis (325).
On quantum actions in electrodynamics (331).
Physical structure of phase space (339).
On the nature of thermal radiation (370).
On the question of quantization of a monatomic gas (384).
Physical reality of light quanta (393).
About Schrödinger's work on wave mechanics (398).
An attempt to synthesize wave and corpuscular mechanics (401).
An attempt to synthesize wave and corpuscular mechanics. Addendum (417).
An attempt to synthesize wave and corpuscular mechanics. Second message (419).
On the history of the discovery of the quantum of action (431).
THEORY OF RELATIVITY
The principle of relativity and the basic equations of mechanics (445).
Kaufman's measurements of b-ray deflection and their implications for Electron dynamics (449).
Addition to discussion of Kaufman measurements (462).
On the dynamics of moving systems (466).
Remarks on the principle of action and reaction in general dynamics (494).
Uniform rotation and Lorentz contraction (498).
ARTICLES AND SPEECHES
About new physics (501).
Theoretical physics (506).
Heinrich Rudolf Hertz (510).
Paul Drude (531).
Helmholtz's merits in theoretical physics (553).
Gottfried Wilhelm Leibniz (550).
To the 25th anniversary of the discovery made by W. Friedrich, P. Knipschg and M. Laue (561).
Memories (564).
Twenty years of work on the physical picture of the world (568).
Origin and influence of scientific ideas (590).
The emergence and gradual development of quantum theory (603).
Unity of the physical picture of the world (613).
The relationship of modern physics to the mechanistic worldview (634).
Scientific autobiography (649).
Academic speeches (664).
APPLICATION
M. Planck and the emergence of quantum physics. L.S. Polak (685).
Comments on one article by M. Planck. A.N. Frumkin (735).
Thermodynamic works of M. Planck. U.I. Frankfurt (737).
M. Planck as a physical chemist. Yu.I. Soloviev (745).
M. Planck's works on the special theory of relativity. AND I. Itenberg, W.I. Frankfurt (754).
Philosophical views of M. Planck. Yu.V. Sachkov, E.M. Chudinov (757).
Bibliography (762).
Name index (781).
Publisher's abstract: This edition of selected works of Max Planck, one of the founders of modern physics, includes articles on thermodynamics, statistical physics, quantum theory, special relativity, as well as general issues of physics and chemistry.
The book is of interest to physicists, chemists, historians of physics and chemistry.
 Nikolai Martynov, who killed M in a duel
Nikolai Martynov, who killed M in a duel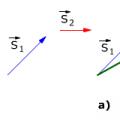 Projections of the displacement vector Module of body displacement over time
Projections of the displacement vector Module of body displacement over time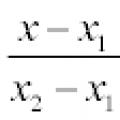 Equation of a line - types of equation of a line: passing through a point, general, canonical, parametric, etc.
Equation of a line - types of equation of a line: passing through a point, general, canonical, parametric, etc.