Download presentation on glassware. Presentation on the topic "assortment of glassware"
slide 2
"Letter on the Benefits of Glass"
They think wrong about glass, Shuvalov, Who revere glass below minerals, There is no less use in it, no less beauty in it. Not infrequently I descend from the Parnassus mountains for that one, And now I return from it to their top. I sing praise before you in delight Not to expensive stones, not to gold, but to glass. M.V. Lomonosov Curator of Moscow University I.I. Shuvalov
slide 3
GLASS -
amorphous-crystalline material obtained from a melt of oxides
slide 4
RAW MATERIALS basic materials (glass-forming) with the help of which acid (Si02, B203, A1203), alkaline earth (CaO, MgO, BaO, ZnO, PbO), alkaline (Na20, K20, Li2 O) oxides are introduced into the glass; - silicon dioxide (silica) auxiliary brighteners bleaching agents dyes molecular colloidal opacifiers
slide 5
The main raw materials for glass production SiO2 determines the basic properties of glass - chemical resistance, thermal, mechanical and optical properties. The mineralogical composition of these materials is heterogeneous. In addition, they are often refractory and cause a defect in the glass - mine stone. B20z - boron oxide, is introduced into the composition: in the form of boric acid, borax or boron-containing minerals. Reduces the coefficient of thermal expansion, the melting temperature, the viscosity of the glass. Increases chemical, thermal stability, strength. AL203 - aluminum oxide, introduced in the form of alumina, feldspar, pegmatite, kaolin, granite - increases the thermal and chemical resistance of glass. MgO, CaO - magnesium oxide and calcium oxide, are introduced using dolomite, limestone, marble, chalk. CaO - increases chemical resistance, speeds up cooking, brightens glass mass; MgO - reduces the coefficient of thermal expansion, the ability to crystallize, increases viscosity, strength, chemical resistance. BaO - barium oxide, is introduced with barium salts. Increases optical properties, promotes uniform coloring of glass mass
slide 6
ZnO - zinc oxide, is introduced with zinc white. Increases chemical and thermal resistance, compressive and expansion strength, refraction, gloss, transparency of glasses. РВО - lead oxide, is introduced with lead litharge or minium. Used to make crystal. Na20 - sodium oxide is introduced into the composition of glass with soda ash, sodium sulfite, soda-potash mixture, when using feldspar rocks. Reduces the cooking temperature, accelerates the process of glass formation, brightens. K20-potassium oxide is introduced using potash, soda-potash mixture, feldspar rocks. It acts similarly to sodium oxide, and also enhances optical properties. Li20 - lithium oxide is introduced with lithium carbonate or with minerals containing lithium - spodumene, etc.
Slide 7
AL203 - aluminum oxide, introduced in the form of alumina, feldspar, pegmatite, kaolin, granite - increases the thermal and chemical resistance of glass. MgO, CaO - magnesium oxide and calcium oxide, are introduced using dolomite, limestone, marble, chalk. CaO - increases chemical resistance, speeds up cooking, brightens glass mass; MgO - reduces the coefficient of thermal expansion, the ability to crystallize, increases viscosity, strength, chemical resistance. BaO - barium oxide, is introduced with barium salts. Increases optical properties, promotes uniform coloring of glass mass. ZnO - zinc oxide, is introduced with zinc white. Increases chemical and thermal resistance, compressive and expansion strength, refraction, gloss, transparency of glasses. K20-potassium oxide is introduced using potash, soda-potash mixture, feldspar rocks. It acts similarly to sodium oxide, and also enhances optical properties. РВО - lead oxide, is introduced with lead litharge or minium. Used to make crystal. Na20 - sodium oxide is introduced into the composition of glass with soda ash, sodium sulfite, soda-potash mixture, when using feldspar rocks. Reduces the cooking temperature, accelerates the process of glass formation, brightens. Li20 - lithium oxide is introduced with lithium carbonate or with minerals containing lithium - spodumene, etc.
Slide 8
Auxiliary raw materials for glass production
Clarifiers - intended for clarification of glass mass (removal of gas bubbles) during cooking. Use substances that decompose with the formation of a large amount of gases - nitrate, ammonium salts, arsenic trioxide. Decolorizers - remove unwanted shades associated with the presence of iron oxides, chromium, etc. in the raw material. By the nature of the action, decolorizers are divided into 2 groups: , which is colored 10 times less intense): saltpeter, arsenic trioxide, antimony; 2. physical - coloring oxides, when used, colors are superimposed and their mutual destruction occurs. For these purposes, oxides of manganese, nickel, selenium, and rare earth metals are used.
Slide 9
Dyes - compounds used to obtain colored glass are divided into 2 groups according to the nature of their action: 1. molecular dyes - metal oxides that dissolve in the glass mass, entering into a compound with it. The resulting color depends on the concentration of the dye and the type of glass. So, cobalt oxide in a ratio of 0.1-0.5% stains glass blue, with a higher content - purple with a reddish tint. 2. colloidal (dispersed) dyes - particles of metals that form colloidal particles with glass, appear during induction (heat treatment). The color depends on the size of the colloidal particles. Colloidal dyes include red dyes - rubies - gold (blood red), copper (with a purple tint), selenium (flame red, with an orange tint), and yellow (silver nitrate).
Slide 10
rare earth elements are also used to color glass to obtain colors: --- yellow - cerium dioxide with titanium dioxide, or samarium oxide; --- lemon yellow - unfired perlite with cerium dioxide or titanium dioxide; --- amber yellow - cerium oxide; --- green-gold-praseodymium oxide; --- violet-lilac - neodymium oxide; --- pink-violet - neodymium oxide with metallic selenium; --- dark pink - erbium oxide; --- red - didim with selenium; --- selenium ruby - selenium and neodymium
slide 11
used to obtain opaque glass. At the same time, depending on the light transmission, glass can be milky (light transmission coefficient of at least 0.6) or opal (less than 0.6). Calcium phosphate, bone meal, cryolite, tin oxide, sodium silicofluoride, as well as compounds of zinc, phosphorus, fluorine, and talc are used as silencers. Silencers
slide 12
Classification of glass types
calcium-sodium-silicate (ordinary) composition: ~70-76% SiO2 ~8-10% CaO, MgO ~8-10% K2O, Na2O K2O > Na2Potassium glasses (the optical properties of glass increase, glass is used for the manufacture of high-quality glassware) K2O
slide 13
calcium-sodium-silicate (ordinary) glass
Slide 14
Ordinary glass products
slide 15
Classification of glass types
crystal glass Lead: crystal glass - not less than 10% PbO low-lead crystal - 18-24% lead crystal -24-30% high-lead crystal - 30-38% PbO optical glass - up to 52% PbO Lead-free barium (not less than 20% barium oxide ), zirconium (8-10% zirconium oxide) lanthanum (4% lanthanum oxide) Glass containing 7-10% barium oxide is called “Bohemian glass”.
slide 16
Crystal products
Slide 17
lead crystal
Slide 18
Lead Free Crystal
Slide 19
crystal skulls
Slide 20
"Bohemian glass"
slide 21
slide 22
slide 23
Classification of glass types
used for technical purposes, as well as for the manufacture of household heat-resistant dishes. Quartz glass consists of pure silica, its composition is similar to rock crystal. Heat-resistant (t pl =1713оС), refractory, chemical and radiation resistant. It is used for glazing spacecraft, instrument parts, sight glasses, fiber optic light guides. heat resistant glass
slide 24
Quartz plates and pipes
Slide 25
Watches with quartz glass
slide 26
Classification of glass types
Borosilicate glass contains up to 12.5% boric anhydride It can be transparent - "merefi" or opaque - "pyrex" It is used for household utensils - tableware, tea and coffee, household, as well as for technical purposes. heat resistant glass
Slide 27
Household borosilicate glassware
Slide 28
Matt blown borosilicate glass
Slide 29
Classification of glass types
Laboratory glass contains 18% aluminum oxide and 4-6% boric anhydride. Possesses high chemical and thermal firmness, transparency, colorlessness. Used for making all kinds of laboratory glassware. heat resistant glass
slide 30
glassware
Slide 31
Classification of glass types
Glass-ceramics are glasses with a crystalline structure, due to which they become resistant to high (up to 300 ° C) temperatures and sudden changes in temperature. They are obtained by introducing metal particles (crystallization centers) into the composition of the glass mass. Slag glass-ceramics are used for construction purposes, and lithium-containing glass-ceramics are used for technical and household products. heat resistant glass
slide 32
Glass-ceramic products are used for microwave
Slide 33
Classification of glass types
when broken, does not give sharp fragments - soda aluminosilicate tempered glass (“Duralex”) Safety glass
slide 34
Slide 35
Classification of glass types
Triplex (safety three-layer) consists of two layers of silicate glass glued with butifol or celluloid in autoclaves under pressure Safety glass
slide 36
Triplex windshields
Slide 37
Classification of glass types
laminated protective glass is silicate glass glued together with polymeric materials in various combinations, silicate glass with organic, polycarbonate or reinforcing films. shock-resistant, withstanding repeated impact of a freely falling body, resistant to penetration (by the butt and an ax blade) bullet-proof (armored glass) Safety glass
Slide 38
Multi-layer bulletproof
Slide 39
Armored glass
Slide 40
armored glass
Slide 41
Classification of glass types
Safety glass is used for glazing buildings, vehicles, aircraft, tanks, and ships. Safety glass
Slide 42
Methods for the production of glass products
Blow Molding Press Blow Rolling
slide 43
Manual blowing with a glass tube using wooden or metal molds in which molding is completed when the blank (bullet) is rotated. This method produces products of any configuration and wall thickness with a smooth and shiny surface. Free blowing (in trade - Guten molding) is also carried out by means of a glass tube, but the products are molded and finally finished mainly in air.
Slide 44
glass blowing
Slide 45
The pressing method produces products that have a simple shape. The pressing process is carried out in metal molds. Pressing is carried out in detachable (consisting of two or three parts) and one-piece forms. pressing Possible molding defects and ways to eliminate them Differences between pressing and blowing
Slide 46
Slide 47
Glass pressing
Slide 48
press-blowing Press-blowing is used to produce glasses and crockery of complex shapes - carafes, bottles, etc.. In this case, the bowl is blown out, and the bottom and leg are pressed and welded to the bowl.
Slide 49
glass blowing
Slide 50
Glass rolling
Slide 51
Decoration methods
applied during the development process (in a hot state, before annealing) - moldings, embankment, crackle, “frosty” glass, natsvet, filigree, etc .; applied to finished products (after annealing) by mechanical (sandblasting, engraving, grinding), chemical (etching) methods, as well as gold, paints, chandeliers.
Slide 52
The range of glass products is subdivided according to the composition of the glass mass, the method of production, purpose, types, styles, sizes and decoration methods. The size of plates, saucers is determined by the upper diameter, mm. The size of glasses and other hollow products - by capacity, cm 3 or l. The size of high products (vases) - in height, mm. cm 3 or l mm

Glasses for red wine - wide, capacious, with and without a stem, usually made of colorless glass to emphasize the color of the wine, capacity cm 3



Glasses for vodka - especially capacious, cm 3. Glasses for cognac - on a short leg, small capacity. The upper part of the glass is necessarily narrowed.


Glasses for soft drinks - tall, elegant, roomy. Glasses for water - wide, low without legs, made of transparent glass, with a capacity of cm 3.









The surface must be smooth, foreign inclusions and other external glass defects are not allowed. The product, placed on a flat surface, should not swing, cutting, sharp edges are not allowed. The lids must fit freely into the product, without noticeable swing. When checking the quality of the product, the quality of processing and decorations is taken into account; product dimensions and volume are checked.

Aesthetic requirements: originality, fashion, high quality processing. Ergonomic requirements: convenience and safety in use. Ease of use depends on the size and design. According to the standard, dishes made of ordinary glass of the same grade are suitable. Crystal products are divided into 1st and 2nd grades.


On crystal products - the content of oxides of lead and barium in percent. Glass products with the inscription "Caution, glass!", "Top", "Do not turn over" are transported. Glass products cannot be stored for a long time in damp rooms, as the transparency of the glass is partially lost, a white coating may form on the surface.

Raw materials for glass production Main raw materials: quartz sand, sodium sulfate, soda, potash, chalk, lead oxides, cullet, etc. Auxiliary materials: illuminators; bleaches; dyes (copper oxide, cobalt oxides, etc.); silencers give the glass an opaque or milky white color. Types of silicate glasses: - ordinary (lime-sodium, lime-calcium); - crystal - has increased brilliance, strong refraction, high transparency; Lead crystal is made up of oxides of silicon, potassium and lead. Barium crystal contains barium oxide. Heat-resistant glass withstands sudden temperature changes, contains boron compounds (up to 12.5%), is highly heat-resistant, and is used for the manufacture of kitchen utensils. Glasses of all types have a high chemical resistance to all chemicals (except hydrofluoric acid).

"World of dishes" - Tasks. Dishes. What did Fedora do to bring the dishes home. Journey into the world of dishes. Help mom set the table. Tea utensils include teapot, cups, saucers. What groups are the dishes divided into. Which country are we going to? Puzzles. Target. Dishes are different. Fedorino grief. For some reason, our friends argued.
"Serving Rules" - Fold the napkin vertically in half. Silver spoon. Place the napkin wrong side up. Flowers. Table. Fold the napkin in quarters. Mug. Cutlery. Learn to use cutlery. Napkins. Plate. Arrange glass. Table linen. Introduce the concept of "table setting".
"Folding napkins" - Styles of the East "Fairy Tale". Sizes and types of napkins in the Middle Ages in France. Tiara. Napkins in history. "Water lily". Napkin decoration. Diagonal. The type, color, fabric and decoration of the napkin can set off the table. "Peacock tail". A beautifully decorated table must be decorated with napkins. French lily.
"Serving the festive table" - Napkins. Refectory bed. Instrument placement options. Napkin folding options. Festive feast. Dinnerware. The art of table setting. Use of napkins. The history of the culture of feasts. Cutlery. Beeswax candles. Lots of food. Table setting options.
"Napkins for table setting" - Open sides. Napkin folding options. A piece of butter. Upper leaves. How to properly use a napkin. Step-by-step implementation of the French lily. Tablecloth. Table fan. Calla. Two types of napkins. Artichokes. Tea drinking. French lily. The history of napkins Breakfast. Napkin folding options.
“Table setting for breakfast” - 5. When serving, the fork is placed to the left of the plate, and the knife to the right. Bon appetit. What is needed for table setting for breakfast: tablecloth, napkins, cutlery, crockery, flowers. Tablespoon. 1. Place the cup on the saucer to the right, near the tip of the knife. Serving utensils. 3. Porridge is served in a half-portion plate.
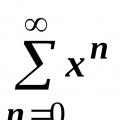 Factoring 6 Composite Numbers Factoring into Prime Factors
Factoring 6 Composite Numbers Factoring into Prime Factors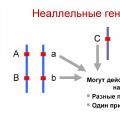 Interaction of non-allelic genes: types and forms The types of interaction of allelic genes include
Interaction of non-allelic genes: types and forms The types of interaction of allelic genes include Presentation “Rules of conduct in the library
Presentation “Rules of conduct in the library