Gas laws. Breathing Boyle-Mariotte's law Boyle-Mariotte's law takes place at constant
The law is formulated as follows: the product of the volume of a given mass of gas and its pressure at a constant temperature is a constant value. Mathematically, this law can be written like this:
P 1 V 1 = P 2 V 2 or PV = const (1)
The following consequences follow from the Boyle-Marriott law: the density and concentration of a gas at a constant temperature is directly proportional to the pressure under which the gas is located:
(2);
 (3) ,
(3) ,
Where d 1 – density, C 1 – gas concentration under pressure P 1; d 2 and C 2 are the corresponding values under pressure P 2 .
Example 1. A gas cylinder with a capacity of 0.02 m 3 contains gas under a pressure of 20 atm. What volume will the gas occupy if the cylinder valve is opened without changing its temperature? Final pressure 1 atm.
Example 2. Compressed air is supplied to a gas holder (gas collection tank) with a volume of 10 m3. How long will it take to pump it up to a pressure of 15 atm if the compressor sucks in 5.5 m 3 of atmospheric air per minute at a pressure of 1 atm? The temperature is assumed to be constant.
Example 3. 112 g of nitrogen under a pressure of 4 atm occupy a volume of 20 liters. What pressure must be applied so that the nitrogen concentration becomes 0.5 mol/l, provided that the temperature remains unchanged?
1.1.2 Gay-Lussac's and Charles' laws
Gay-Lussac found that at constant pressure, with an increase in temperature of 1°C, the volume of a given mass of gas increases by 1/273 of its volume at 0°C.
Mathematically, this law is written:
 (4)
,
(4)
,
Where V- volume of gas at temperature t°С, a V 0 – volume of gas at 0°C.
Charles showed that the pressure of a given mass of gas, when heated by 1°C at a constant volume, increases by 1/273 of the pressure that the gas has at 0°C. Mathematically, this law is written as follows:
 (5)
,
(5)
,
where P 0 and P are gas pressures, respectively, at temperatures 0С and tС.
When replacing the Celsius scale with the Kelvin scale, the connection between them is established by the relation T = 273 + t, the formulas of Gay-Lussac's and Charles's laws are significantly simplified.
Gay-Lussac's Law: at constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature:
 (6)
.
(6)
.
Charles's Law: at constant volume, the pressure of a given mass of gas is directly proportional to its absolute temperature:
 (7)
.
(7)
.
From the laws of Gay-Lussac and Charles it follows that at constant pressure the density and concentration of a gas are inversely proportional to its absolute temperature:
 (8)
,
(8)
,
 (9) .
(9) .
Where d 1 and C 1 - density and concentration of gas at absolute temperature T 1, d 2 and C 2 are the corresponding values at absolute temperature T 2 .
Example 4. At 20ºC the volume of gas is 20.4 ml. What volume will the gas occupy when it is cooled to 0°C if the pressure remains constant?
Primep 5. At 9°C, the pressure inside the oxygen cylinder was 94 atm. Calculate how much the pressure in the cylinder increased if the temperature rose to 27ºC?
Example 6. Density of chlorine gas at 0ºС and pressure 760 mm Hg. Art. equal to 3.220 g/l. Find the density of chlorine, taking it as an ideal gas, at 27ºС at the same pressure.
Example 7. Under normal conditions, the concentration of carbon monoxide is 0.03 kmol/m3. Calculate at what temperature the mass of 10 m 3 of carbon monoxide will be equal to 7 kg?
Combined Boyle-Mariotte-Charles-Gay-Lussac law.
The formulation of this law: for a given mass of gas, the product of pressure and volume divided by absolute temperature is constant for all changes occurring in the gas. Mathematical notation:
 (10)
(10)
where V 1 is the volume and P 1 is the pressure of a given mass of gas at absolute temperature T 1 , V 2 - volume and P 2 - pressure of the same mass of gas at absolute temperature T 2.
One of the most important applications of the unified gas law is “bringing the volume of gas to normal conditions.”
Example 8. Gas at 15°C and pressure 760 mmHg. Art. occupies a volume of 2 liters. Bring the volume of gas to normal conditions.
To facilitate such calculations, you can use the conversion factors given in the tables.
Example 9. In the gasometer above the water there is 7.4 liters of oxygen at a temperature of 23°C and a pressure of 781 mm Hg. Art. The water vapor pressure at this temperature is 21 mmHg. Art. What volume will the oxygen in the gasometer occupy under normal conditions?
Change in one of the macroscopic parameters of a substance of a certain mass - pressure R, volume V or temperature t - causes changes to other parameters.
If all the quantities characterizing the state of the gas change simultaneously, then it is difficult to establish any definite patterns experimentally. It’s easier to first study processes in which mass and one of three parameters - R,V or t - remain unchanged. Quantitative relationships between two parameters of a gas of the same mass with a constant value of the third parameter are called gas laws.
Boyle-Mariotte law
The first gas law was discovered by the English scientist R. Boyle (1627-1691) in 1660. Boyle’s work was called “New Experiments Concerning an Air Spring.” Indeed, gas behaves like a compressed spring; this can be verified by compressing air in a regular bicycle pump.
Boyle studied the change in gas pressure as a function of volume at constant temperature. The process of changing the state of a thermodynamic system at a constant temperature is called isothermal (from the Greek words isos - equal, therme - heat). To maintain a constant temperature of a gas, it is necessary that it can exchange heat with a large system in which a constant temperature is maintained - a thermostat. Atmospheric air can serve as a thermostat if its temperature does not change noticeably during the experiment.
Boyle observed the change in the volume of air trapped in a long curved tube by a column of mercury (Fig. 3.6, a). Initially, the mercury levels in both legs of the tube were the same and the air pressure was equal to atmospheric pressure (760 mm Hg). While adding mercury to the long elbow of the tube, Boyle noticed that the volume of air was halved when the difference in levels in both elbows turned out to be equal h = 760 mm, and, consequently, the air pressure doubled (Fig. 3.6, b). This led Boyle to the idea that the volume of a given mass of gas and its pressure are inversely proportional.
A) b)
Further observations of the change in volume when adding different portions of mercury confirmed this conclusion.
Independently of Boyle, somewhat later, the French scientist E. Marriott (1620-1684) came to the same conclusions. Therefore, the found law was called the Boyle-Mariotte law. According to this law, the pressure of a given mass (or amount) of gas at a constant temperature is inversely proportional to the volume of the gas:  .
.
If p 1 - gas pressure at volume V 1 , And p 2 - its pressure at volume V 2 , That
 (3.5.1)
(3.5.1)
It follows that p 1 V l = p 2 V 2 , or
 (3.5.2)
(3.5.2)
at t = const.
The product of the pressure of a gas of a given mass and its volume is constant if the temperature does not change.
This law is valid for any gases, as well as for mixtures of gases (for example, air).
You can verify the validity of the Boyle-Mariotte law using the device shown in Figure 3.7. The sealed corrugated vessel is connected to a pressure gauge that records the pressure inside the vessel. By rotating the screw you can change the volume of the vessel. The volume can be judged using a ruler. By changing the volume and measuring the pressure, you can see that equation (3.5.2) is satisfied.

Like other physical laws, the Boyle-Mariotte law is approximate. At pressures several hundred times greater than atmospheric pressure, deviations from this law become significant.
On a graph of pressure versus volume, each state of a gas corresponds to one point.
Isotherms
The process of changing gas pressure depending on volume is depicted graphically using a curve called an isotherm (Fig. 3.8). The gas isotherm expresses the inverse relationship between pressure and volume. A curve of this kind is called a hyperbola. Different isotherms correspond to different constant temperatures, since a higher temperature at the same volume corresponds to a higher pressure*. Therefore, the isotherm corresponding to a higher temperature t2, lies above the isotherm corresponding to the lower temperature t 1.
* This will be discussed in more detail later.

The Boyle-Mariotte law is one of the fundamental laws of physics and chemistry, which relates changes in pressure and volume of gaseous substances. Using our calculator it is easy to solve simple problems in physics or chemistry.
Boyle-Mariotte law
The isothermal gas law was discovered by an Irish scientist Robert Boyle, who conducted experiments on gases under pressure. Using a U-shaped tube and ordinary mercury, Boyle established a simple principle that at any given time the product of pressure and volume of a gas is constant. Speaking in dry mathematical language, the Boyle-Mariotte law states that at constant temperature the product of pressure and volume is constant:
To maintain a constant ratio, quantities must change in different directions: by how many times one quantity decreases, by the same number of times another increases. Consequently, the pressure and volume of a gas are inversely proportional and the law can be rewritten as follows:
P1×V1 = P2×V2,
where P1 and V1 are the initial values of pressure and volume, respectively, and P2 and V2 are the final values.
Application of the Boyle-Mariotte law
The best illustration of the manifestation of the law discovered by Boyle is the immersion of a plastic bottle under water. It is known that if a gas is placed in a cylinder, then the pressure on the substance will be determined only by the walls of the cylinder. It's another matter when it is a plastic bottle that easily changes its shape. On the surface of the water (pressure 1 atmosphere), a closed bottle will retain its shape, but when immersed to a depth of 10 m, a pressure of 2 atmospheres will act on the walls of the vessel, the bottle will begin to shrink, and the volume of air will decrease by half. The deeper the plastic container is immersed, the less volume the air inside it will occupy.
This simple demonstration of the gas law illustrates an important point for many divers. If on the surface of the water an air cylinder has a capacity of 20 liters, then when diving to a depth of 30 m, the air inside will be compressed three times, therefore, the air for breathing at such a depth will be three times less than on the surface.
Beyond the diving theme, the Boyle-Marriott law in action can be observed in the process of compressing air in a compressor or in the expansion of gases when using a pump.
Our program is an online tool that makes it easy to calculate the proportion for any gas isothermal process. To use the tool, you need to know any three quantities, and the calculator will automatically calculate the required one.
Examples of how the calculator works
School task
Let's consider a simple school problem in which you need to find the initial volume of a gas if the pressure changes from 1 to 3 atmospheres and the volume decreases to 10 liters. So, we have all the data for the calculation that needs to be entered into the appropriate cells of the calculator. As a result, we find that the initial volume of gas was 30 liters.
More about diving
Let's remember a plastic bottle. Let's imagine that we immersed a bottle filled with 19 liters of air to a depth of 40 m. How will the volume of air on the surface change? This is a more difficult problem, but only because we need to convert depth into pressure. We know that at the surface of water the atmospheric pressure is 1 bar, and when immersed in water the pressure increases by 1 bar every 10 m. This means that at a depth of 40 m the bottle will be under a pressure of approximately 5 atmospheres. We have all the data for the calculation, and as a result we will see that the volume of air on the surface will increase to 95 liters.
Conclusion
The Boyle-Marriott law occurs quite often in our lives, so you will undoubtedly need a calculator that automates calculations using this simple proportion.
The basic laws of ideal gases are used in technical thermodynamics to solve a number of engineering problems in the process of developing design and technological documentation for aviation equipment and aircraft engines; their production and operation.
These laws were originally obtained experimentally. Subsequently, they were derived from the molecular kinetic theory of the structure of bodies.
Boyle–Mariotte law establishes the dependence of the volume of an ideal gas on pressure at constant temperature. This dependence was derived by the English chemist and physicist R. Boyle in 1662, long before the advent of the kinetic theory of gas. Independently of Boyle, the same law was discovered by E. Marriott in 1676. Law of Robert Boyle (1627 - 1691), an English chemist and physicist who established this law in 1662, and Edme Mariotte (1620 - 1684), a French physicist who established this law in 1676: the product of the volume of a given mass of an ideal gas and its pressure is constant at a constant temperature or.
The Boyle–Mariotte law is called and states that at constant temperature, gas pressure is inversely proportional to its volume.
Let us assume at a constant temperature of a certain mass of gas:
V 1 – volume of gas at pressure R 1 ;
V 2 – volume of gas at pressure R 2 .
Then, according to the law, we can write
Substituting the value of the specific volume into this equation and taking the mass of this gas T= 1kg, we get
p 1 v 1 =p 2 v 2 or pv= const .(5)
Gas density is the reciprocal of its specific volume:
then equation (4) will take the form
that is, the densities of gases are directly proportional to their absolute pressures. Equation (5) can be considered as a new expression of the Boyle–Mariotte law which can be formulated as follows: the product of pressure and the specific volume of a certain mass of the same ideal gas for its different states, but at the same temperature, is a constant value.
This law can be easily obtained from the basic equation of the kinetic theory of gases. Replacing the number of molecules per unit volume in equation (2) with the ratio N/V (V– volume of a given mass of gas, N– number of molecules in volume) we obtain
Since for a given mass of gas the values N And β are constant, then at constant temperature T=const for an arbitrary amount of gas, the Boyle–Mariotte equation will have the form
pV = const, (7)
and for 1 kg of gas
pv = const.
Let us depict graphically in the coordinate system R – v change in gas state.
For example, the pressure of a given mass of gas with a volume of 1 m 3 is equal to 98 kPa, then, using equation (7), we determine the pressure of a gas with a volume of 2 m 3
Continuing the calculations, we obtain the following data: V(m 3) equals 1; 2; 3; 4; 5; 6; respectively R(kPa) equals 98; 49; 32.7; 24.5; 19.6; 16.3. Using these data we build a graph (Fig. 1).
Rice. 1. Dependence of ideal gas pressure on volume at
constant temperature
The resulting curve - a hyperbola obtained at a constant temperature - is called an isotherm, and a process occurring at a constant temperature is called isothermal. The Boyle–Mariotte law is approximate and at very high pressures and low temperatures is unacceptable for thermotechnical calculations.
Gay-Lussak Law determines the dependence of the volume of an ideal gas on temperature at constant pressure. (Law of Joseph Louis Gay-Lussac (1778 - 1850), a French chemist and physicist who first established this law in 1802: the volume of a given mass of ideal gas at constant pressure increases linearly with increasing temperature, that is , where is the specific volume at; β is the coefficient of volume expansion equal to 1/273.16 per 1 o C.) The law was established experimentally in 1802 by the French physicist and chemist Joseph Louis Gay-Lussac, after whom it was named. Exploring the thermal expansion of gases experimentally, Gay-Lussac discovered that at constant pressure, the volumes of all gases increase when heated almost equally, i.e., with an increase in temperature by 1 ° C, the volume of a certain mass of gas increases by 1/273 of the volume that this mass gas occupied at 0°C.
The increase in volume when heated by 1 °C by the same amount is not accidental, but seems to be a consequence of the Boyle-Mariotte law. Initially, the gas is heated at a constant volume by 1 °C, its pressure increases by 1/273 of the initial one. Then the gas expands at a constant temperature, and its pressure decreases to the initial one, and its volume increases by the same amount. Denoting the volume of a certain mass of gas at 0°C by V 0 , and at temperature t°C in V t Let's write the law as follows:
Gay-Lussac's law can also be represented graphically.
Rice. 2. Dependence of the volume of an ideal gas on temperature at constant
pressure
Using equation (8) and taking the temperature equal to 0°C, 273°C, 546°C, we calculate the volume of gas equal to, respectively V 0 , 2V 0 , 3V 0 . Let us plot the gas temperatures along the abscissa axis on a certain conventional scale (Fig. 2), and the gas volumes corresponding to these temperatures along the ordinate axis. By connecting the obtained points on the graph, we obtain a straight line representing the dependence of the volume of an ideal gas on temperature at constant pressure. This line is called isobar, and the process occurring at constant pressure is isobaric.
Let us turn once again to the graph of changes in gas volume versus temperature. Let's continue the straight line until it intersects with the x-axis. The intersection point will correspond to absolute zero.
Let us assume that in equation (8) the value V t= 0, then we have:
but since V 0 ≠ 0, therefore, where does t= – 273°C. But – 273°C=0K, which is what needed to be proven.
Let us represent the Gay-Lussac equation in the form:
Remembering that 273+ t=T, and 273 K=0°C, we get:
Substituting the value of the specific volume into equation (9) and taking T=1 kg, we get:
Relation (10) expresses Gay-Lussac’s law, which can be formulated as follows: at constant pressure, the specific volumes of identical masses of the same ideal gas are directly proportional to its absolute temperatures. As can be seen from equation (10), Gay-Lussac's law states that that the quotient of the specific volume of a given mass of gas divided by its absolute temperature is a constant value at a given constant pressure.
The equation expressing the Gay-Lussac law has the general form
and can be obtained from the basic equation of the kinetic theory of gases. Equation (6) will be represented in the form
at p=const we obtain equation (11). Gay-Lussac's law is widely used in technology. Thus, based on the law of volumetric expansion of gases, an ideal gas thermometer was built for measuring temperatures in the range from 1 to 1400 K.
Charles's Law establishes the dependence of the pressure of a given mass of gas on temperature at a constant volume. The law of Jean Charles (1746 - 1823), a French scientist who established this law for the first time in 1787, and refined by J. Gay-Lussaccombe in 1802: the pressure of an ideal gas of constant mass and volume increases linearly when heated, that is, where R o – pressure at t= 0°C.
Charles determined that when heated in a constant volume, the pressure of all gases increases almost equally, i.e. with an increase in temperature by 1 °C, the pressure of any gas increases by exactly 1/273 of the pressure that a given mass of gas had at 0 °C. Let us denote the pressure of a certain mass of gas in a vessel at 0°C by R 0 , and at temperature t° through p t. When the temperature rises by 1°C, the pressure increases by, and when the temperature increases by t°C pressure increases by. Pressure at temperature t°Equal to initial plus pressure increase or
Formula (12) allows you to calculate the pressure at any temperature if the pressure at 0°C is known. In engineering calculations, the equation (Charles' law) is very often used, which is easily obtained from relation (12).
Since, and 273 + t = T or 273 K = 0°C = T 0
At constant specific volume, the absolute pressures of an ideal gas are directly proportional to the absolute temperatures. Reversing the middle terms of the proportion, we get
Equation (14) is an expression of Charles’s law in general form. This equation can be easily derived from formula (6)
At V=const we obtain the general equation of Charles’s law (14).
To plot the dependence of a given mass of gas on temperature at a constant volume, we use equation (13). Let, for example, at a temperature of 273 K = 0°C, the pressure of a certain mass of gas is 98 kPa. According to the equation, the pressure at temperatures of 373, 473, 573 °C will respectively be 137 kPa (1.4 kgf/cm2), 172 kPa (1.76 kgf/cm2), 207 kPa (2.12 kgf/cm2). Using these data we build a graph (Fig. 3). The resulting straight line is called an isochore, and the process occurring at a constant volume is called isochoric.
Rice. 3. Dependence of gas pressure on temperature at constant volume
Boyle-Mariotte Law (Isotherm), one of the basic gas laws that describes isothermal processes in ideal gases. It was established by scientists R. Boyle in 1662 and E. Marriott in 1676 independently of each other during an experimental study of the dependence of gas pressure on its volume at a constant temperature.
According to the Boyle-Mariotte law at constant temperature (T=const), the Volume (V) of a given mass (m) of an ideal gas is inversely proportional to its pressure (p):
pV = const = C at T=const and m=const
The constant C is proportional to the mass of the gas (number of moles) and its absolute temperature. In other words: the product of the volume of a given mass of an ideal gas and its pressure is constant at a constant temperature. Boyle-Mariotte's law holds strictly for an ideal gas. For real gases, the Boyle-Mariotte law is satisfied approximately. Almost all gases behave as ideal gases at not too high pressures and not too low temperatures.
The Boyle-Mariotte law follows from the kinetic theory of gases, when the assumption is made that the sizes of molecules are negligible compared to the distance between them and there is no intermolecular interaction. At high pressures, it is necessary to introduce corrections for the forces of attraction between molecules and for the volume of the molecules themselves. Like the Clayperon equation, the Boyle-Mariotte law describes the limiting case of the behavior of a real gas, more accurately described by the van der Waals equation. The application of the law can be approximately observed in the process of compressing air by a compressor or as a result of the expansion of gas under the piston of a pump when pumping it out of a vessel.
A thermodynamic process that occurs at a constant temperature is called isothermal. Its image on the graph (Fig. 1) is called an isotherm.
Fig.1
Gay-Lussac's law. Isobar
In 1802, the French scientist J. Gay-Lussac experimentally discovered the dependence of gas volume on temperature at constant pressure. The data is the basis of Gay-Lussac's gas law.
The formulation of Gay-Lussac's law is as follows: for a given mass of gas, the ratio of the volume of the gas to its temperature is constant if the pressure of the gas does not change. This relationship is written mathematically as follows:
V/T=const, if P=const and m=const
This law can be approximately observed when gas expands when it is heated in a cylinder with a movable piston. Constant pressure in the cylinder is ensured by atmospheric pressure on the outer surface of the piston. Another manifestation of Gay-Lussac's law in action is the balloon. Gay-Lussac's law is not observed in the region of low temperatures close to the temperature of liquefaction (condensation) of gases.
The law is valid for an ideal gas. It works well for rarefied gases, which are close to ideal in their properties. The gas temperature must be high enough.
Graphically, this dependence in V-T coordinates is depicted as a straight line extending from the point T=0. This straight line is called an isobar. Different pressures correspond to different isobars. The process of changing the state of a thermodynamic system at constant pressure is called isobaric (Fig. 2 graph of an isobaric process).

Fig.2
Charles's law. Isochora
In 1787, the French scientist J. Charles experimentally discovered the dependence of gas pressure on temperature at constant volume. The data is the basis of Charles' gas law.
The formulation of Charles's law is as follows: for a given mass of gas, the ratio of gas pressure to its temperature is constant if the volume of the gas does not change. This relationship is written mathematically as follows:
P/T=const, if V=const and m=const
This law can be approximately observed when gas pressure increases in any container or in an electric light bulb when heated. The isochoric process is used in constant-volume gas thermometers. Charles's law is not observed in the region of low temperatures close to the temperature of liquefaction (condensation) of gases.
The law is valid for an ideal gas. It works well for rarefied gases, which are close to ideal in their properties. The gas temperature must be high enough. The process must be very slow
Graphically, this dependence in P-T coordinates is depicted as a straight line extending from the point T=0. This straight line is called an isochore. Different isochores correspond to different volumes. The process of changing the state of a thermodynamic system at a constant volume is called isochoric. Fig. 3 (graph of an isochoric process).
 Albert Einstein short biography
Albert Einstein short biography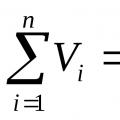 Dalton's law for a mixture of gases: examples of problem solving
Dalton's law for a mixture of gases: examples of problem solving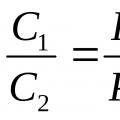 Breathing Boyle-Mariotte's law Boyle-Mariotte's law takes place at constant
Breathing Boyle-Mariotte's law Boyle-Mariotte's law takes place at constant