What is the content of Dalton's law? Dalton's law for a mixture of gases: examples of problem solving
A gas mixture is in a state of equilibrium if the concentrations of the components and its state parameters throughout the volume have the same values. In this case, the temperature of all gases included in the mixture is the same and equal to the temperature of the mixture T cm.
In an equilibrium state, the molecules of each gas are scattered evenly throughout the entire volume of the mixture, that is, they have their own specific concentration and, therefore, their own pressure R i, Pa, which is called partial . It is defined as follows.
The partial pressure is equal to the pressure of a given component, provided that it alone occupies the entire volume intended for the mixture at the mixture temperature T cm .
According to the law of the English chemist and physicist Dalton, formulated in 1801, the pressure of a mixture of ideal gases p cm equal to the sum of the partial pressures of its components p i :
Where n– number of components.
Expression (2) is also called law of partial pressures.
3.3. The reduced volume of a component of a gas mixture. Amag's Law
By definition, the reduced volume i th component of the gas mixture V i, m3, is the volume that this one component could occupy, provided that its pressure and temperature are equal to the pressure and temperature of the entire gas mixture.
The law of the French physicist Amag, formulated around 1870, states: the sum of the reduced volumes of all components of a mixture is equal to the volume of the mixtureV cm :
 , m 3. (3)
, m 3. (3)
3.4. Chemical composition of the gas mixture
The chemical composition of the gas mixture can be specified three different ways.
Consider a gas mixture consisting of n components. The mixture occupies volume V cm, m 3, has mass M cm, kg, pressure R cm, Pa and temperature T cm, K. Also, the number of moles of the mixture is N cm, mole. At the same time, the mass of one i th component m i, kg, and the number of moles of this component ν i, mole.
It's obvious that:
 , (4)
, (4)
 . (5)
. (5)
Using Dalton’s law (2) and Amag’s law (3) for the mixture under consideration, we can write:
 , (6)
, (6)
 , (7)
, (7)
Where R i– partial pressure i th component, Pa; V i– reduced volume i th component, m3.
Unambiguously, the chemical composition of a gas mixture can be specified either by mass, or mole, or volume fractions of its components:
 , (8)
, (8)
 , (9)
, (9)
 , (10)
, (10)
Where g i , k i And r i– mass, mole and volume fractions i th component of the mixture, respectively (dimensionless values).
It's obvious that:
 ,
,
 ,
, . (11)
. (11)
Often in practice, the chemical composition of a mixture is not specified in fractions i th component, and its percentage.
For example, in heating engineering it is approximately accepted that dry air consists of 79 volume percent nitrogen and 21 volume percent oxygen.
Percent i The th component in the mixture is calculated by multiplying its share by 100.
For example with dry air we will have:
 ,
,
 . (12)
. (12)
Where  And
And  – volume fractions of nitrogen and oxygen in dry air; N 2 and O 2 – designation of the volume percentage of nitrogen and oxygen, respectively, % (vol.).
– volume fractions of nitrogen and oxygen in dry air; N 2 and O 2 – designation of the volume percentage of nitrogen and oxygen, respectively, % (vol.).
Note:
1)The mole fractions of an ideal mixture are numerically equal to the volume fractions:k i = r i . Let's prove it.
Using the definition of volume fraction(10)and Amag’s law (3) we can write:
 ,
(13)
,
(13)
WhereV i – reduced volumeith component, m 3
;
ν
i – number of molesith component, mol;
 – volume of one moleith component at mixture pressure p cm and mixture temperature T cm , m 3
/mol.
– volume of one moleith component at mixture pressure p cm and mixture temperature T cm , m 3
/mol.
From Avogadro’s law (see paragraph 2.3 of this appendix) it follows that at the same temperature and pressure, one mole of any gas (mixture component) occupies the same volume. In particular, at T cm and p cm it will be some volumeV 1 , m 3 .
This allows us to write the equality:
 .
(14)
.
(14)
Substituting(14)V(13)we get what we need:
 .
(15)
.
(15)
2)The volume fractions of the components of a gas mixture can be calculated by knowing their partial pressures. Let's show it.
Let's consideri-th component of an ideal gas mixture in two different states: when it is at its partial pressure p i ; when it occupies its reduced volumeV i .
The equation of state of an ideal gas is valid for any of its states, in particular, for the two mentioned above.
In accordance with this, and taking into account the definition of specific volume, we can write:
 ,
(16)
,
(16)
 ,
(17)
,
(17)
WhereR i – gas constantith component of the mixture, J/(kg K).
After dividing both parts(16)And(17)on each other we get the required:
 .
(18)
.
(18)
From(18)it can be seen that the partial pressures of the components of the mixture can be calculated from its chemical composition, with a known total pressure of the mixture p cm :
 .
(19)
.
(19)
Formulation of laws
Law on the total pressure of a mixture of gases
Law on the solubility of gas mixture components
At a constant temperature, the solubility in a given liquid of each of the components of the gas mixture located above the liquid is proportional to their partial pressure.
Limits of applicability
Both Dalton's laws are strictly satisfied for ideal gases. For real gases, these laws are applicable provided that their solubility is low and their behavior is close to that of an ideal gas.
History of discovery
The law of addition of partial pressures was formulated in 1801. At the same time, the correct theoretical justification, based on molecular kinetic theory, was made much later.
Notes
Wikimedia Foundation. 2010.
See what "Dalton's Laws" are in other dictionaries:
DALTON'S LAWS- (Dalton Dalton): the first law, the total pressure of a mixture of ideal gases that do not chemically interact with each other is equal to the sum of the partial (see) individual gases that make up the mixture, i.e., the pressures that each gas would produce in ... ... Big Polytechnic Encyclopedia
Dalton's laws- discovered by the English physicist and chemist J. Dalton (1766 1844) in 1801 and 1803. 1) the pressure of a mixture of chemically non-interacting ideal gases is equal to the sum of the partial pressures. Let's apply to real gases at temperatures and pressures... ... Concepts of modern natural science. Glossary of basic terms
The basic laws of chemistry can be divided into qualitative and quantitative. Contents 1 Qualitative laws 1.1 I. Gibbs’ phase law ... Wikipedia
DALTON'S LAWS- (more correctly Dalton, Dalton). 1. The law of multiple ratios, discovered by D., is that elements are included in a chemical. connections in ratios that are always multiples of certain prime numbers. So, if they have water, then for one part by weight of hydrogen... ... Great Medical Encyclopedia
DALTON'S LAWS: 1) the pressure of a mixture of gases that do not chemically interact with each other is equal to the sum of their partial pressures; 2) the solubility of a component of a gas mixture in a given liquid at a constant temperature is proportional to the partial... ... Big Encyclopedic Dictionary
1) the pressure of a mixture of chemically non-interacting ideal gases is equal to the sum of the partial pressures. Approximately applicable to real gases at temperatures p and pressures far from critical. 2) At constant rate of solubility in a given liquid... ... Physical encyclopedia
1) the pressure of a mixture of chemically non-interacting ideal gases is equal to the sum of the partial pressures. Approximately applicable to real gases at temperatures and pressures far from critical. 2) At constant temperature solubility in a given ... Physical encyclopedia
DALTON'S LAWS: 1) the pressure of a mixture of gases that do not chemically interact with each other is equal to the sum of their partial pressures; 2) the solubility of a component of a gas mixture in a given liquid at a constant temperature is proportional to the partial... ... encyclopedic Dictionary
Describe processes occurring in equilibrium “liquid solution-vapor” systems under the influence of temperature or pressure. Contents 1 Konovalov’s first law 2 Konovalov’s second law ... Wikipedia
This article or section needs revision. Please improve the article in accordance with the rules for writing articles. The whole ... Wikipedia
At the end of the 18th and in the first half of the 19th century, scientists from different countries actively studied the behavior of gaseous, liquid and solid matter under various external conditions, basing their research on ideas about the atomic and molecular structure of matter. One of these scientists was the British Law for a mixture of gases, which currently bears his name, is discussed in this article.
Special conditions
Before formulating Dalton's law for a mixture of gases, one of the concepts should be understood. This is very important, since this law is valid only for such a substance. We are talking about an ideal gas. What is it?
An ideal gas is one for which the following requirements apply:
- the sizes of molecules and atoms in it are so small that they can be considered material points with zero volume;
- molecules and atoms do not interact with each other.
Thus, an ideal gas is a collection of material points moving randomly. The speed of their movement and mass uniquely determine the temperature of the entire mixture. The pressure that the test substance exerts on the walls of the vessel depends on such macroscopic parameters as temperature, volume of the vessel and the number of molecules.
For such a gas model the equality is valid:
It is called and combines pressure (P), temperature (T), volume (V) and the amount of substance in moles (n). The value of R is the proportionality coefficient, which is equal to 8.314 J/(K*mol).
The surprising thing about this formula is that it does not include a single parameter that would depend on the chemical nature of molecules and atoms.
Partial pressure
Dalton's law for a mixture of ideal gases presupposes knowledge of one more macroscopic parameter - partial pressure.
Let's assume that there is some mixture consisting of 2 components, for example, H 2 and He. This mixture is in a vessel of a specific volume and creates a certain pressure on its walls. Since hydrogen molecules and helium atoms do not interact with each other, then for any calculations of macroscopic characteristics both components can be considered independently of each other.
The partial pressure of a component is the pressure that it creates independently of the other components of the mixture, occupying the volume provided to it. In the example under consideration, we can talk about the partial pressure of H 2 and the same characteristics for He. This value is expressed in pascals and is denoted for the i-th component as Pi.
Gas mixtures and Dalton's law

John Dalton, studying various volatiles, including water vapor, at different temperatures and pressures, came to the following conclusion: the pressure of a mixture of absolutely any similar substances in any proportions is equal to the sum of the partial pressures of all its components. This formulation is called Dalton’s law for the pressure of a mixture of gases and is written as follows:
Here P tot is the total pressure of the mixture.
This fairly simple law is true only for ideal gas mixtures, the components of which do not react chemically with each other.
Another formulation of Dalton's law

Dalton's law for a mixture of gases can be expressed not only in terms of partial pressures, but also in terms of the mole fractions of each component. We obtain the corresponding formula.
Since each component behaves independently of the others in the gas mixture, then the equation of state can be written for it:
This equation is valid for each i-th component, since for all of them the temperature T and volume V are the same. The value n i is the number of moles of component i in the mixture.
Let us now express the partial pressure and divide it by the total pressure of the entire mixture, then we get:
P i /P tot = n i *R*T / V / (n *R*T/V) = n i /n
Here n is the total amount of substance in the entire mixture. It can be obtained by summing all n i . The ratio n i /n is called the mole fraction of component i in the mixture. It is usually denoted by the symbol x i. In terms of mole fractions, Dalton's law is written as follows:
Often represented as atomic percentages of the components in a mixture. For example, 21% O 2 in the air means that its mole fraction is 0.21, that is, every fifth air molecule is oxygen.
Application of the considered law to solve the problem

It is known that a gas mixture of oxygen and nitrogen is under a pressure of 5 atmospheres in a cylinder. Knowing that it contains 10 moles of nitrogen and 3 moles of oxygen, it is necessary to determine the partial pressure of each substance.
To answer the question of the problem, let’s first find the total amount of substance:
n = n N2 + n O2 = 10 + 3 = 13 mol
x N2 = n N2 /n = 10/13 = 0.7692
x O2 = n O2 /n = 3/13 = 0.2308
Using the formula of Dalton’s law through the mole fraction of a component, we calculate the partial pressure of each gas in the cylinder:
P N2 = 5*0.7692 = 3.846 atm.
P O2 = 5*0.2308 = 1.154 atm.
As can be seen from the obtained figures, the sum of these pressures will give 5 atmospheres. The partial pressure of each gas is directly proportional to its mole fraction in the mixture.
 Albert Einstein short biography
Albert Einstein short biography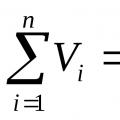 Dalton's law for a mixture of gases: examples of problem solving
Dalton's law for a mixture of gases: examples of problem solving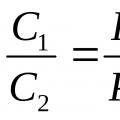 Breathing Boyle-Mariotte's law Boyle-Mariotte's law takes place at constant
Breathing Boyle-Mariotte's law Boyle-Mariotte's law takes place at constant